CGF166 update: Results from the phase 1/2 study of the hearing loss drug are now available.
Developing story…
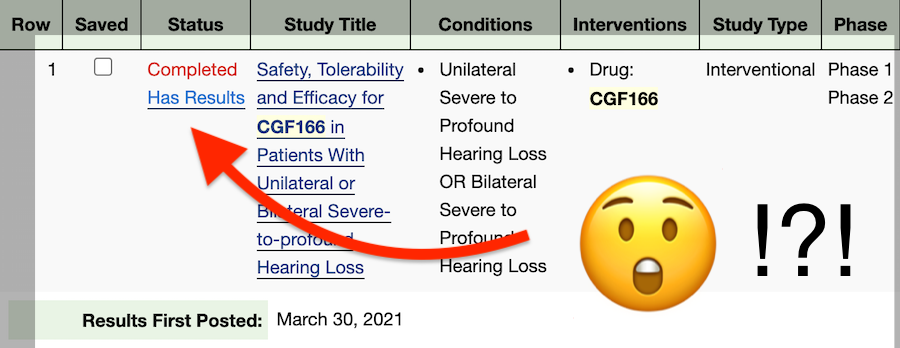
Two days ago, on March 30, 2021, the official study record of CGF166 was updated on ClinicalTrials.gov.
This update was not accompanied by a press release from Novartis nor any media coverage.
However, this “surprise” update had two important words: has results.
An unexpected update
These results came out of nowhere…
For anyone learning about CGF166 for the first time, here is some quick background info on the “mysterious” Novartis gene therapy drug candidate for hearing loss:
A Case Study: Using Regenerative Medicine to Treat Hearing Loss
In 1999, scientists singled out a gene called atonal as a “master switch” for turning on the growth of inner ear hair cells, which pick up sound waves and translate them into electrical signals in the brain. Humans are born with hair cells, but the atonal switch flips off at birth. Any subsequent damage to hair cells is permanent.
In collaboration with a biotech company, GenVec Inc, NIBR researchers have developed an experimental gene therapy called CGF166 to restore hearing function by regenerating hair cells. It consists of a viral vector carrying the atonal gene. The vector has been altered with the aim of making it harmless and is injected directly into the inner ear. CGF166 is now being tested in a limited number of patients with severe-to-profound hearing loss.
SOURCE: an old brochure from the Novartis Institute for Biological Research (NIBR), circa 2016.
Sounds promising, right?
Absolutely.
But that material was written 5 years ago and… since then – aside from the early buzz and media coverage on this holy grail treatment – CGF166 updates have been incredibly rare.
In fact, before today… the last credible update we got on CGF166 was from over a year ago when, on February 21, 2020, BioCentury pointed out that Novartis had “yet to publicly report data from its Phase I/II trial of gene therapy CGF166.”
SOURCE: Regenerative medicine for hearing loss makes quiet progress, BioCentury, https://www.biocentury.com/article/304491, dated February 21, 2020.
Well, now it looks like they (quietly) have.
The results were uploaded to the study record of the phase I/II clinical trial. Here is the official description:
The goal of the study was to evaluate the safety, tolerability, and the potential ability of CGF166 delivered through IL-infusion to improve hearing. CGF166 is a recombinant adenovirus 5 (Ad5) vector containing a cDNA encoding the human Atonal transcription factor (Hath1).
This study evaluated the safety, tolerability, and potential efficacy of CGF166 and the associated delivery procedures in patients with severe-to-profound unilateral or bilateral hearing loss. Eligible patients were required to have documented, non-fluctuating hearing loss.
SOURCE: https://clinicaltrials.gov/ct2/show/NCT02132130, last updated March 30, 2021.
CGF166 study results: Part 1
These results do not show all the data and numbers, but we expect to post a follow-up once we have some experts review them.
Here is the direct link to the study results that were uploaded on March 30, 2021: ClinicalTrials.gov Identifier: NCT02132130 – Study Results
It’s important to keep in mind that these results are NOT the same thing as an announcement or press release from the company. For that reason, there is no accompanying commentary or explanation of what these results mean.
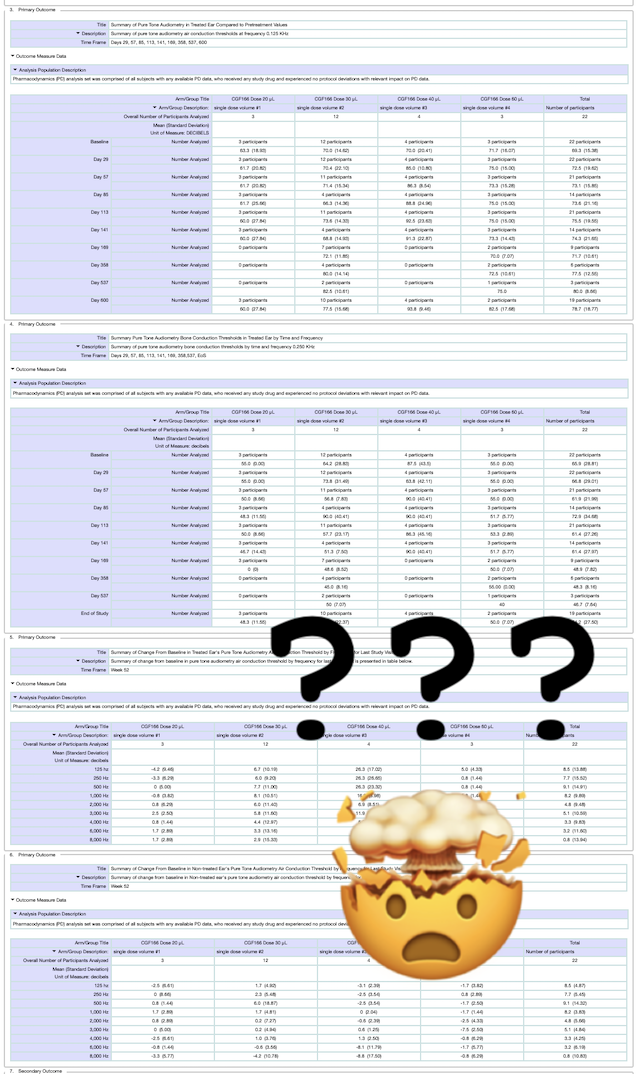 This can be frustrating, but here at Hearing Loss Treatment Report we are currently working on a follow-up post that will provide a clear idea of what these numbers mean for people with hearing loss.
This can be frustrating, but here at Hearing Loss Treatment Report we are currently working on a follow-up post that will provide a clear idea of what these numbers mean for people with hearing loss.
This is a developing story…
I wish we had more for you.
This is all we have, for now.
We will continue to monitor what is happening with CGF166 and post an update as soon as we know more. (The best way to get updates is by subscribing to our email newsletter – which we’ll be sending out very soon!)

For now, we decided to share the link to these “raw” results because we believe in sharing early and sharing often. Information ASAP. Before the official press releases, before company Twitter announcements, before the media outlets, before before before.
And so, for that reason, we didn’t want to sit and wait on this long-awaited study result update just so we could organize it into a neatly-wrapped package with in-depth commentary.
That’s not our style.
“First”
The reason we believe in this post-first-question-later approach is because an increasingly large number of patients, organizations, and industry professionals use this site as a shortcut to the very latest hearing loss treatment updates.
Think of this site like a secret source of some of the fastest, earliest updates in the world of hearing loss drug development. And it’s meant to help inform people in situations like this: where this CGF166 phase I/II results story was missed, ignored, or simply too early (and thus unknown) to the mainstream media.
Which is also why you might want to subscribe to our email updates list… so you can get as-fast-as-we-can-find-them updates on experimental hearing restoration drugs and milestones… plus, sneak previews of what potential “cures” are really around the corner in 2021, 2022, and beyond…
But it’s not for everyone.
Questions? Comments? Corrections? Feedback:
Send an email to michael@urgentresearch.com and say hello.