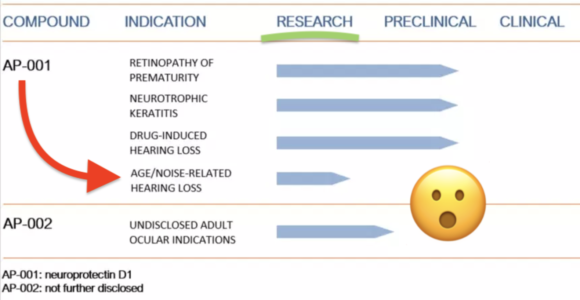
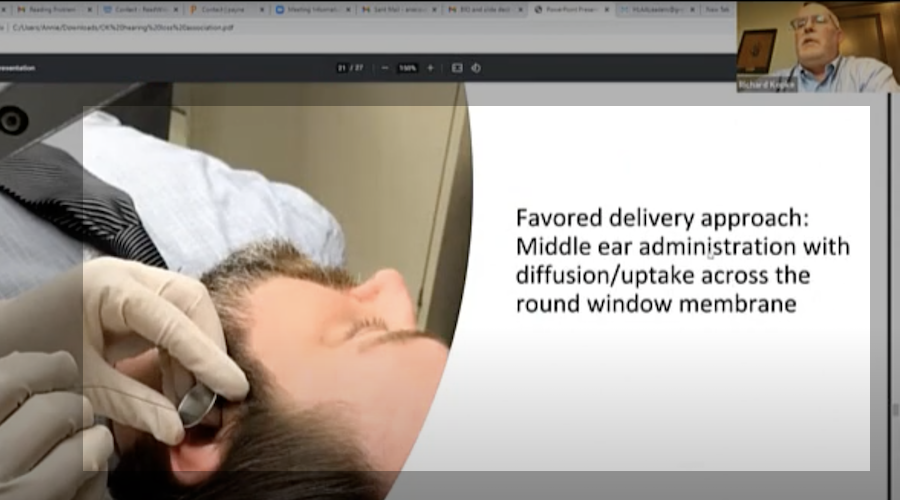
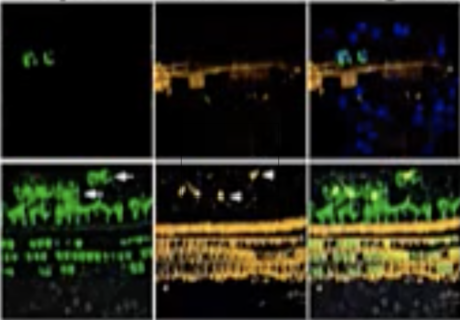
First details on NS101, a new pipeline candidate for sensorineural hearing loss (previously “undisclosed indication”), from Neuracle Science.
A new phase 1/2 clinical trial of NS101 for the treatment of hearing loss was added to clinicaltrials.gov on February 8, 2024. The trial, titled A Phase 1b/2a, Study Evaluating the Safety, PK/PD and Efficacy of NS101 in Healthy Volunteers and SSNHL Patients, lists a start date of January 19, 2024. The study record and public details only emerged more than a month later, however.
Here is what we know so far:
This new trial is currently recruiting participants and the sponsor, Neuracle Science, plans to enroll a total of 118 participants across 16 locations.
Important point: although the study is evaluating NS101 for SSNHL (sudden sensorineural hearing loss), the testing on healthy volunteers is not without significance. The drug’s potential applications extend beyond sudden/acute situation, and it is not limited to the “rescue therapy” category of treatment.
From the official clinical trial record (NCT06249919):
“NS101 is an anti FAM19A5 antibody expected to play as a synapse organizer and reversing synapse dysfunction in various neurological diseases.”
What else do we know about NS101—and its “regenerative” potential?
NS101 is an investigational new treatment that promotes nerve synapses and nerve regeneration. It’s being developed by Neuracle Science Co., Ltd., in collaboration with Samsung and Korean University. This broad applicability to nerve synapses and regeneration means that NS101 is also being researched to help with degenerative neurological diseases such as Alzheimer’s disease, ALS, and acute spinal cord injury. This means that NS101 may also help with other synaptopathies as well as different kinds of sensorineural hearing loss (not just the sudden sensorineural hearing loss being studied in this particular trial).
This is also supported by clues from the official trial record—note the provided keywords:

Last year, on January 26, 2023, the company shared a press release outlining positive results of its first-in-human phase 1 clinical trial of NS101. That trial showed that the treatment had a good safety profile and “robust FAM19A5 target engagement.”
“Neuracle Science (CEO: Dr. Jae Young Seong) announced top-line results from the Phase 1 study of NS101, an investigational monoclonal antibody for the treatment of neurological diseases. NS101 binds to and neutralizes FAM19A5/TAFA5, a potential target for synaptopathy.”
At that time, Neuracle Science also shared that they planned “to initiate further clinical trials with an undisclosed indication…” Based on this phase 1/2 trial development, that undisclosed indication mentioned last year appears to have been hearing loss (plus tinnitus—but more about that later).
The Neuracle Science company website also has some more information, though its website has not yet been updated to cover the hearing loss indication(s). Note: This is not uncommon for small/private biotech companies, as they are usually too busy focusing on the research to update their pipeline snapshot. The company might post an update soon — hopefully one with a deeper dive into the mechanism of NS101 for various forms of sensorineural hearing loss.
Quick background on Neuracle Science, the company behind NS101:
According to last year’s press release, “Neuracle Science is a clinical-stage biotech company specializing in the development of new drugs for the treatment of neurological diseases.”
Here is some additional information about Neuracle Science:

What type of drug is NS101?
- IV infusion
- Doses = 15 mg/kg or 30 mg/kg
- Frequency = Every second week over the course of 12 weeks
- Participants will be followed for up to 20 weeks
In addition to testing for safety and tolerability, this study will look at whether NS101 is effective in improving hearing in people with sudden sensorineural hearing loss who have not benefited from treatment with oral steroids. This clinical trial is set up to be placebo-controlled and double-blinded.
Researchers will be looking for improvements in these outcomes:
- Hearing capacity (via Pure Tone Audiometry, ABR, DPOAE, ECochG)
- Speech discrimination (via SRT, SDS)
- Tinnitus (via THI, VAS)
Looking further ahead, the anticipated completion date for the study is June 19, 2025.
Here is the link to the official study record for A Phase 1b/2a, Double-blinded, Placebo-controlled, Multiple Doses, 2 Step-up Study Evaluating the Safety, Tolerability, PK/PD and Efficacy of Systemic NS101 in Healthy Volunteers and SSNHL Patients—last updated on February 8, 2024:
https://clinicaltrials.gov/study/NCT06249919
How to get email updates on the development of NS101
If you want updates on NS101 from Neuracle Science — including the progress of this new clinical trial — sign up for the Hearing Loss Treatment Report email newsletter.
It’s free, your information is kept private, and you can unsubscribe at any time with a single click. As a subscriber, you get early access to “first look” updates like this one — before they are added to the front page of this website (or anywhere else).
Expect between 1-2 emails per week… but only if something interesting and treatment-related is happening.