Short but sweet update:
We now have an estimated completion date for the PIPE-505 phase 2a clinical trial: June 12, 2021.
Details below:
- The PIPE-505 study record was updated on March 29, 2021.
- The study completion date was changed from March 2021 [Anticipated] to June 2021 [Anticipated].
- The June 12, 2021 date [Estimated] was calculated using information found within the study record and its history of changes, including: a) the submission date on which the Study Status was changed from “Recruiting” to “Active, not recruiting” and, b) the maximum follow-up Time Frame (“3 months after drug administration”) as detailed in the Outcome Measures section.
- For a more conservative completion date, replace the last submitted update to Study Status date with the more recent last submitted update date of March 26, 2021. This gives us June 24, 2021.
- Taken together, we are looking at a range of June 12 – June 24, with June 12th as the one to mark on your calendar.
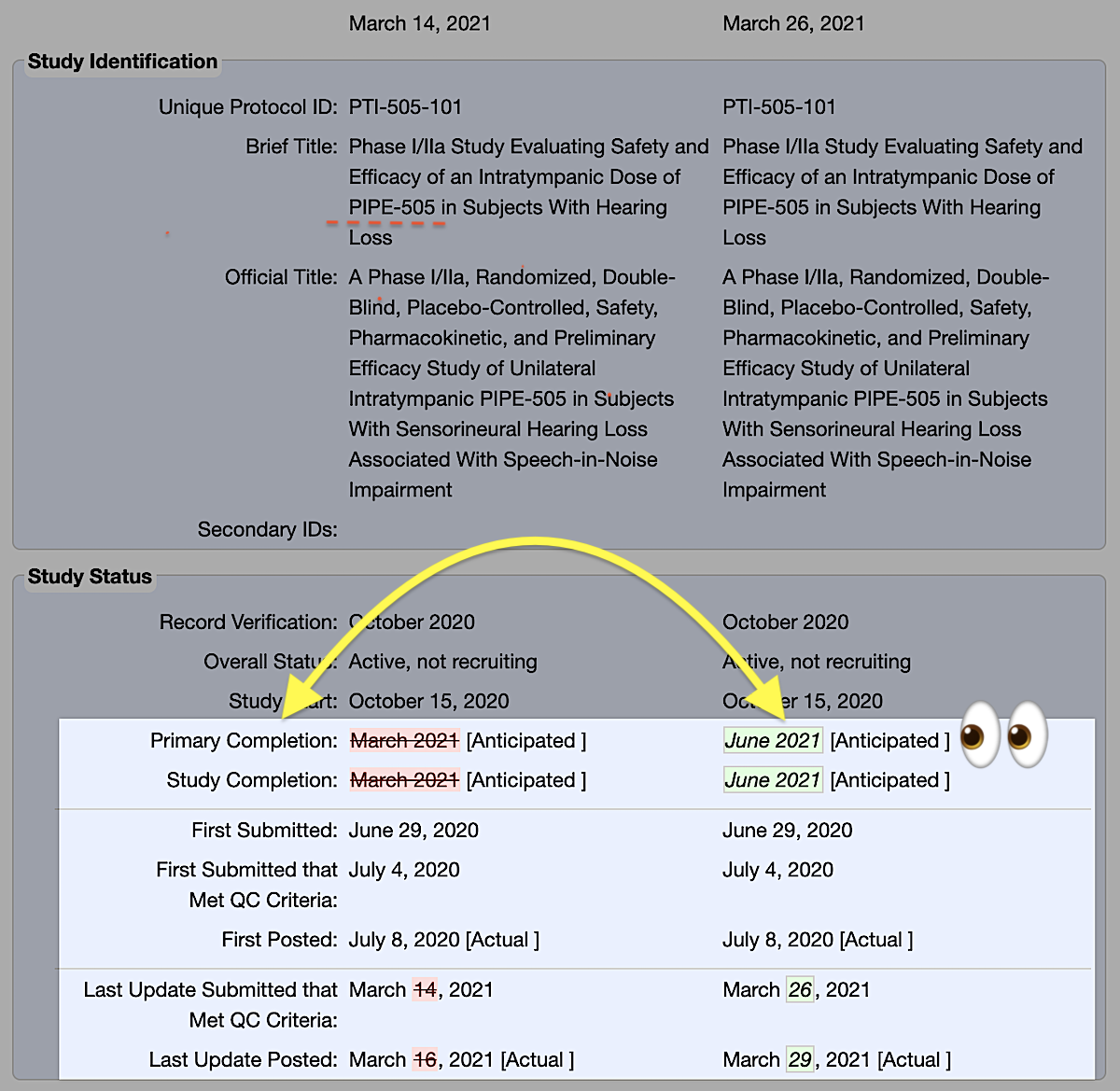
Source: History of Changes for Study: NCT04462198 (Phase I/IIa Study Evaluating Safety and Efficacy of an Intratympanic Dose of PIPE-505 in Subjects With Hearing Loss)
That’s all for now.
We will probably have the next PIPE-505 update for you before June 15th (and/or whenever Pipeline Therapeutics makes an announcement).
For ongoing PIPE-505 updates and news of other upcoming hearing loss treatments, sign up for the free email newsletter. No spam, no promotional emails, privacy respected. Between 1-3 emails per week (but only when something interesting is happening).