FX-345 program will “enable the company to clinically evaluate increased cochlear coverage across range of doses in multiple patient populations,” according to a recent presentation uploaded to the Frequency Therapeutics website.
Background:
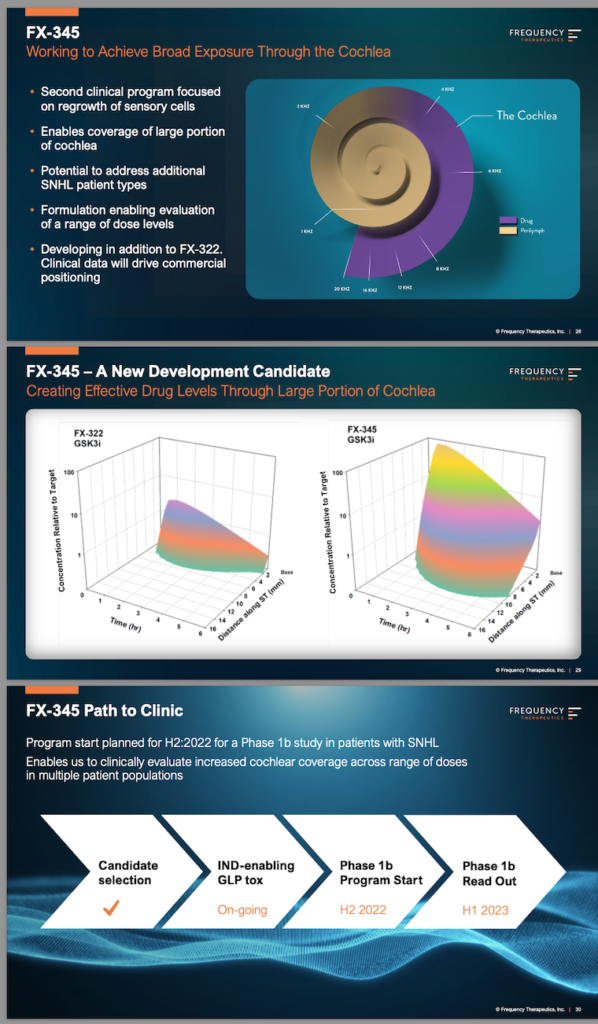
FX-345 is Frequency Therapeutics’ second clinical program for sensorineural hearing loss, focused on the regrowth of sensory cells. The drug is being developed in addition to FX-322 (important distinction). Based on what we have been told so far, the big idea behind FX-345 — and its potential to restore hearing in SNHL patients — seems to be related to increased cochlear coverage. This particular aspect of the new drug candidate is repeated throughout the three-page FX-345 section of the presentation:
- “Broad Exposure Through the Cochlea”
- “Enables coverage of large portion of cochlea”
- “Through Large Portion of Cochlea”
That’s the idea, anyway. But, as the presentation also points out:
- “Clinical data will drive commercial positioning”
A good reminder and also a good reason to look forward to the second half of the year, when the phase 1 trial is slated to begin…
Sneak preview of the full presentation (pages 28 to 30):
Below is a link to the full “Corporate Presentation – January 22” from Frequency Therapeutics. It is a 57-page PDF document but go to page 28 if you only want to read the FX-345 section (three pages, total):
https://investors.frequencytx.com/static-files/82a80eb8-8cd7-443b-a9c7-7c19d0d1b99d
What happens next?
For updates on the development status of FX-345 — and other promising new treatments currently in development for sensorineural hearing loss — subscribe to the newsletter (email updates). It’s free, your information is kept private and not shared or sold to third parties… no spam or sponsored content… Instead, it’s a way to get “nothing but new treatment updates”, and only when something important happens.