https://www.forbes.com/sites/williamhaseltine/2024/07/02/gene-therapy-to-restore-hearing-a-new-portal/
gene therapy
Mogrify and Astellas announce collaboration to conduct research on in vivo regenerative medicine approaches to address sensorineural hearing loss
https://mogrify.co.uk/mogrify-and-astellas-announce-collaboration-to-conduct-research-on-in-vivo-regenerative-medicine-approaches-to-address-sensorineural-hearing-loss/
Gene Therapy for Hearing Loss on the Horizon
https://journals.lww.com/thehearingjournal/Fulltext/2022/01000/Gene_Therapy_for_Hearing_Loss_on_the_Horizon.1.aspx
Discovery of new hearing loss gene in humans [GAS2]
https://medicalxpress.com/news/2021-05-discovery-genetic-loss-illuminates-ear.html
Discovery of new hearing loss gene in humans – the first one known to affect the mechanical properties of inner ear support cells
FGF signaling system for hearing restoration
Researchers at the University of Utah’s Mansour Lab are studying the Fibroblast Growth Factor signaling pathway with the long-term goal of harnessing developmental signals to drive hearing restoration.
“Our results will contribute new knowledge to the long-term goal of harnessing developmental signals to drive hearing restoration.”
The project has received funding from the National Institute on Deafness and Other Communication Disorders (NIDCD) and will continue through January 2022.
According to investigators, the results “will facilitate future efforts to manipulate the FGF signaling system for hearing restoration.”
First, they will use mouse models to investigate the role of the Fibroblast Growth Factor signaling pathway and its critical role in the inner ear.
But the findings could uncover key development signals that could potentially be commandeered to restore hearing function.

The team is being led by Dr. Suzanne L. Mansour (Molecular Biology Program – FGFs and Inner Ear Development, Mouse models of hearing loss and restoration), who has been studying these FGF signals since the early 2000s.
Here is the full abstract and public health relevance statement:
Regulation of inner ear development by FGF signals and effectors
Mansour, Suzanne L.
University of Utah, Salt Lake City, UT, United StatesAbstract
Morphogenesis of the inner ear epithelium requires coordinated deployment of several signaling pathways and disruptions cause abnormalities of hearing and/or balance. With the advent of cochlear implantation to treat hearing loss even in cases of inner ear malformation, it is critical to understand exactly how such malformations affect the auditory ganglia and innervation. Also, in light of the intense focus on in vitro generation of inner ear cell types for transplantation and in vivo manipulation of developmental signaling molecules to promote differentiation of various inner ear cells for hearing restoration, elucidating the roles and regulation of such signals and their effectors governing otic differentiation and morphogenesis are necessary to advance treatment. The genes encoding FGF3 and FGF10, ligands that signal through FGFR2b and FGFR1b, are expressed dynamically throughout otic development in both epithelial and ganglion domains. Studies conducted by the Mansour Lab of both conventional Fgf3 and Fgf10 conditional knockout mice and those expressing a doxycycline-inducible ligand trap (dnFGFR2b) that rapidly inhibits signaling through both FGFR1b and FGFR2b, showed that Fgf3 and Fgf10 are not required in the placode lineage for otocyst formation, but are required subsequently for otocyst patterning, neuroblast maintenance, epithelial proliferation and both vestibular and cochlear morphogenesis. Furthermore, the first genome wide analyses of otocyst mRNA revealed FGFR2b/1b signaling targets that define novel candidates for genes involved in otic morphogenesis and function. This proposal has two Aims addressing the hypotheses that 1) FGFR2b/1b signaling is required continuously for both otic neuroblast specification and maintenance, and that at later stages, mesenchymal signaling, as well as that in the epithelial and ganglion domains, is required for cochlear epithelial differentiation and ganglion maintenance and 2) FGFR2b/1b downstream target genes mediate some or all of the effects of FGFR2b/1b signaling on otic morphogenesis and gangliogenesis. To determine the early role of FGFR2b/1b signaling in otic ganglion formation and its later role in epithelial differentiation and ganglion maintenance, DOX-induced ubiquitous and CRE-limited expression of dnFGFR2b will be employed and morphology and molecular markers of otic patterning, proliferation and survival in both tissues will be assessed. To determine the roles of downstream targets of FGFR2b/1b signaling, two genes encoding transcription factors that are activated by FGFR2b/1b signaling and one gene encoding a BMP signaling regulator that is repressed by FGFR2b/1b signaling will be studied. Otic conditional mutants will be generated for each gene, and their morphologic and functional development will be assessed. In addition, the extent to which the BMP regulator contributes to the dnFGFR2b phenotypes and the effects of overexpressing the BMP regulator will be assessed. The results will contribute new knowledge that will facilitate future efforts to manipulate the FGF signaling system for hearing restoration.
Public Health Relevance
Permanent hearing loss caused by malformation of the inner ear or congenital or progressive loss of its sensory or neural cells affects up to one third of individuals by the age of 80 and generates significant social and healthcare costs. In this proposal, we use mouse models to investigate the role of the Fibroblast Growth Factor signaling pathway in forming the inner ear epithelium and neurons. Our results will contribute new knowledge to the long-term goal of harnessing developmental signals to drive hearing restoration.
For updates on Mansour’s FGF signaling system (and other hearing restoration research/treatments) sign up for free email updates (our newsletter) here.
References
Project #: 1R01DC019127-01
DB-301: Update on Decibel Therapeutics’ cochlear hair cell regeneration program for hearing loss
An unofficial update on Decibel Therapeutics’ secretive gene therapy program to treat sensorineural hearing loss by regenerating cochlear outer hair cells…
First, the official story, followed by a big “unofficial” update on “DB-301″…
Officially:
Laurence Reid, CEO of Decibel Therapeutics, had this to say during a recent presentation at the 20th Annual Needham Virtual Healthcare Conference – from April 15, 2021:
Laurence Reid: We have an earlier stage program with respect to the cochlea. The goal is to drive differentiation of the supporting cell, through an immature hair cell, and then on particularly to either generate the outer hair cell or potentially the inner hair cell – for treating different types of patient populations with different cellular bases for their hearing loss.
Here is a slide from the presentation:
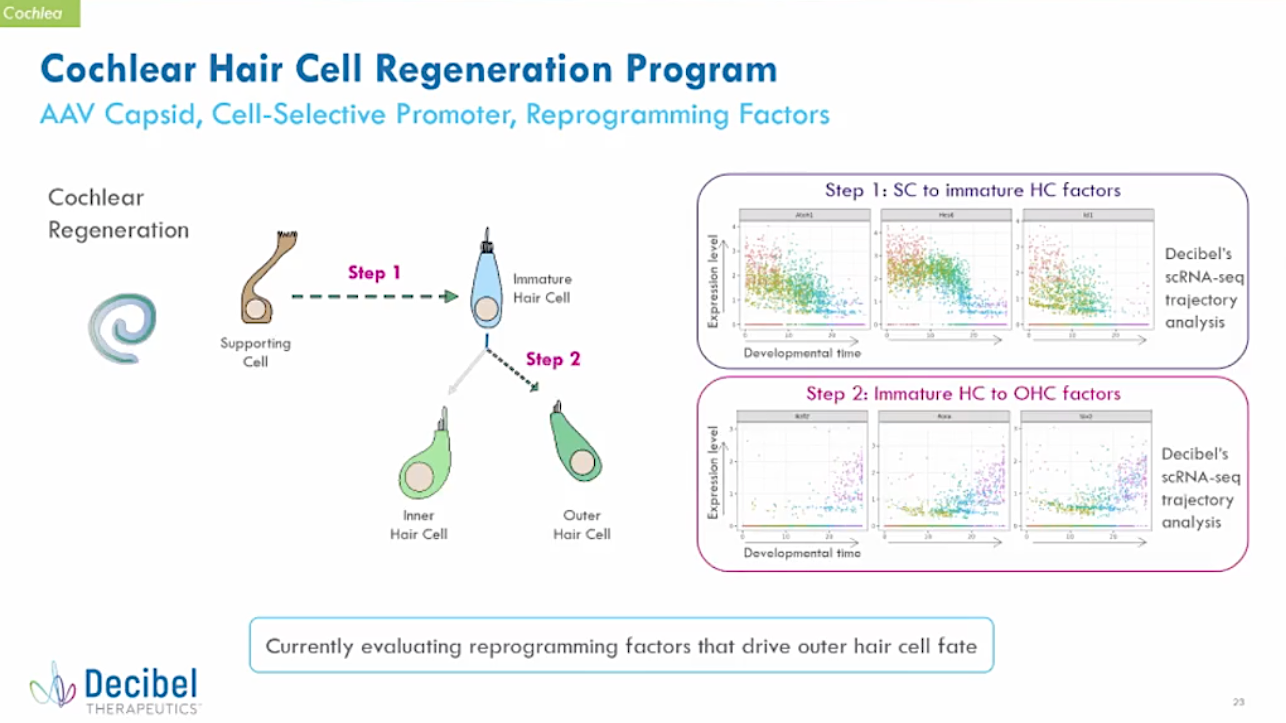
Officially, we also have some background information thanks to a recent Decibel Therapeutics, Inc SEC filing (emphasis ours):
Cochlear Hair Cell Regeneration
Age-related hearing loss and noise-induced hearing loss affect millions of people in the United States and Europe. Research has shown that the degree of hearing loss in these populations is best predicted by the amount of outer hair cell loss. We believe that restoring outer hair cells could restore hearing in these individuals. In our cochlear hair cell regeneration program, we are designing an AAV-based gene therapy that utilizes cell-selective expression of reprogramming factors to convert supporting cells into outer hair cells. We are currently conducting preclinical in vitro and in vivo rodent studies to evaluate the cell-selectivity of certain proprietary promoters and the ability of certain reprogramming factors that may drive an outer hair cell fate.
In that same filing, we learn that Decibel Therapeutics plans to announce the targets for this cochlear hair cell regeneration program in 2022. Excerpt and pipeline snapshot:
In addition, we are advancing our cochlear hair cell regeneration program to treat acquired hearing loss by regenerating cochlear outer hair cells. We plan to announce the targets for our cochlear hair cell regeneration program in 2022.

However, we might not need to wait until 2022 for that update, because…
Unofficially:
Our independent research has uncovered what appear to be details related to Decibel’s “Cochlear Hair Cell Regeneration Program” (a.k.a. the gene therapy program which could potentially be given the name “DB-301”)…
“DB-301”?
That’s our unofficial code name, inferred from Decibel’s naming scheme (“AAV.RF301” or “AAV.301” + “DB” = DB-301).
From a Decibel Therapeutics corporate overview presented during Citi’s 15th Annual BioPharma Virtual Conference:
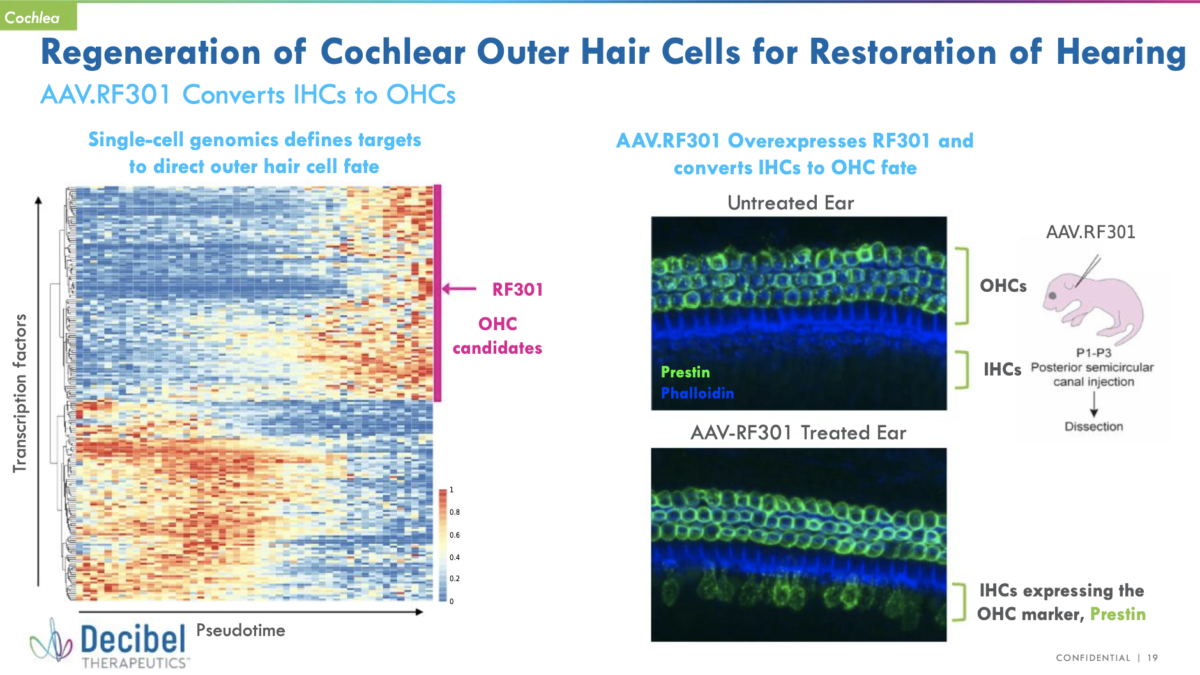
As you can see, this slide looks like a perfect match for the cochlear hair cell regeneration program. (Which is why we’re calling this an “early update” on the program.)
Confidential?
The presentation has the word “confidential” written on page 19.
However, the source of this information – a PDF document – is currently available on Decibel’s website. (It is indexed by search engines and accessible to the public. We have archived it digitally as well, for record-keeping purposes.)

Intentional?
Furthermore, within this PDF – on page 19, in very, very, very tiny text – is a list of what appear to be outer hair cell candidate targets (copy+pasted):
Hdac3 Kdm4a Mtpn Htatip2 Dnmt3a Mlxip Foxo3
oxn3 Prnp Sall1 Pknox2 Klf9 Rora Six2 Trps1 Actn4 Mef2a Pdcd11 Nfkb1 Hlcs Zfp410 Banp Zbtb7b Esrra E2f3 Zfp740 Zkscan3 Creb3l1 Ighm Zfp324 Arnt Lig1 Elk3 Zbtb40 Crem Smarca2 Nfatc2 Rest Nfib Zfp608 Nfix Smad7 Mafk Adarb1 Irf6 Nacc2 Rorb Bhlhe40 Ikzf2 Jun Zfp423 Nr3c1 Tppp Cat Zfp637 Zfp777 Kmt2a Nr2f2 Zfp592 Pura Zfp365 Zbtb46 Zfp654 BC005561 Zfp516 Cic Zhx3 Rxra Zfp106 Zfp277 Thra Tmem33 Zc3h7a Srrm3 Pds5a Zbtb4 Ube2k Ctbp2 Id4 Srebf1 Nfat5 Ddit3 Sall3 Mdm2 Srebf2 Gtf2i Prdx5 Smap2 Zbtb7a Zfp618 Gata3 Irf9 Mef2d Taf1 Zfp523 Kdm2a Abcf2 Zfp398 Zfp638 Hmg20a Msi2 Nmral1 Tead1 ead2 H2afy Irx3 Ezh2 Rfxap Sox9 Tgif2 Egr4 Egr3 Npas4 Sox11 Fhl2 Prox1 Sox2 Cers2 Gm10093 Dazap1 Dnajc21 Isl1 Ran Ebf1 Atoh1 Hes6 Rpl35 Rps10 Psma6 Akr1a1 Barhl1 Smarca5 Ruvbl1 U2af1 Gtf2a2 Zfp428 Ssbp3 Zfp326 Nuak1 Pknox1 Cyb5r1 Tceal5 Msra Cers6 Zmat4 Bcl11b Npdc1 Bcl2 Ybx1 Stub1 Zmat2 Zfp667 Yeats4 Tfdp2 Hnrnpa1 Tfdp1 Rbm17 Hmgn3 Lhx3 Cers4 Ugp2 Bax Mrps25 H1fx Nap1l1 Pax2 Traf4 Mcm6 Neurod6 Rab2a Cd59a Ptcd1 Klf7 Las1l Cdk2ap1 Mycl Nono Zfp330 Insm1 Irx2 Id1 Id2 Magoh Nr2f6 Zbtb20 Diablo Gar1 Snrpb2 Rps4x Zmiz1 Hnrnpc Nr2f1 Rbpj Lsm6 Hmgb2 Tbpl1
Is this a short list of targets that Decibel is planning to choose from and announce in 2022?
Time will tell.
But, taken together, all this information leads us to believe that Decibel Therapeutics could have an earlier update for us about all these “unofficial” details (including the name “DB-301”, which is just one possibility).
References
https://wsw.com/webcast/needham107/deci/2205522
http://www.decibeltx.com/wp-content/uploads/ARO-2021_Inner-Ear-Tropism_Poster-1.pdf
https://www.sec.gov/Archives/edgar/data/1656536/000156459021016167/0001564590-21-016167.txt
http://www.decibeltx.com/wp-content/uploads/Decibel-Corp-Overview-Q320-CITI-conference.pdf
COMMENT: This article was made available to email subscribers several days early. If you want early access to updates like this one, join the email updates list. It’s free, no spam, and your information is kept private.
BREAKING: CGF166 Clinical Trial Results Posted Online…
CGF166 update: Results from the phase 1/2 study of the hearing loss drug are now available.
Developing story…
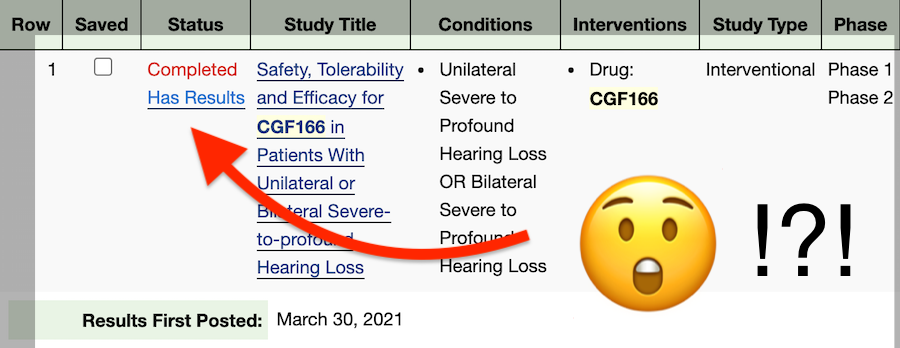
Two days ago, on March 30, 2021, the official study record of CGF166 was updated on ClinicalTrials.gov.
This update was not accompanied by a press release from Novartis nor any media coverage.
However, this “surprise” update had two important words: has results.
An unexpected update
These results came out of nowhere…
For anyone learning about CGF166 for the first time, here is some quick background info on the “mysterious” Novartis gene therapy drug candidate for hearing loss:
A Case Study: Using Regenerative Medicine to Treat Hearing Loss
In 1999, scientists singled out a gene called atonal as a “master switch” for turning on the growth of inner ear hair cells, which pick up sound waves and translate them into electrical signals in the brain. Humans are born with hair cells, but the atonal switch flips off at birth. Any subsequent damage to hair cells is permanent.
In collaboration with a biotech company, GenVec Inc, NIBR researchers have developed an experimental gene therapy called CGF166 to restore hearing function by regenerating hair cells. It consists of a viral vector carrying the atonal gene. The vector has been altered with the aim of making it harmless and is injected directly into the inner ear. CGF166 is now being tested in a limited number of patients with severe-to-profound hearing loss.
SOURCE: an old brochure from the Novartis Institute for Biological Research (NIBR), circa 2016.
Sounds promising, right?
Absolutely.
But that material was written 5 years ago and… since then – aside from the early buzz and media coverage on this holy grail treatment – CGF166 updates have been incredibly rare.
In fact, before today… the last credible update we got on CGF166 was from over a year ago when, on February 21, 2020, BioCentury pointed out that Novartis had “yet to publicly report data from its Phase I/II trial of gene therapy CGF166.”
SOURCE: Regenerative medicine for hearing loss makes quiet progress, BioCentury, https://www.biocentury.com/article/304491, dated February 21, 2020.
Well, now it looks like they (quietly) have.
The results were uploaded to the study record of the phase I/II clinical trial. Here is the official description:
The goal of the study was to evaluate the safety, tolerability, and the potential ability of CGF166 delivered through IL-infusion to improve hearing. CGF166 is a recombinant adenovirus 5 (Ad5) vector containing a cDNA encoding the human Atonal transcription factor (Hath1).
This study evaluated the safety, tolerability, and potential efficacy of CGF166 and the associated delivery procedures in patients with severe-to-profound unilateral or bilateral hearing loss. Eligible patients were required to have documented, non-fluctuating hearing loss.
SOURCE: https://clinicaltrials.gov/ct2/show/NCT02132130, last updated March 30, 2021.
CGF166 study results: Part 1
These results do not show all the data and numbers, but we expect to post a follow-up once we have some experts review them.
Here is the direct link to the study results that were uploaded on March 30, 2021: ClinicalTrials.gov Identifier: NCT02132130 – Study Results
It’s important to keep in mind that these results are NOT the same thing as an announcement or press release from the company. For that reason, there is no accompanying commentary or explanation of what these results mean.
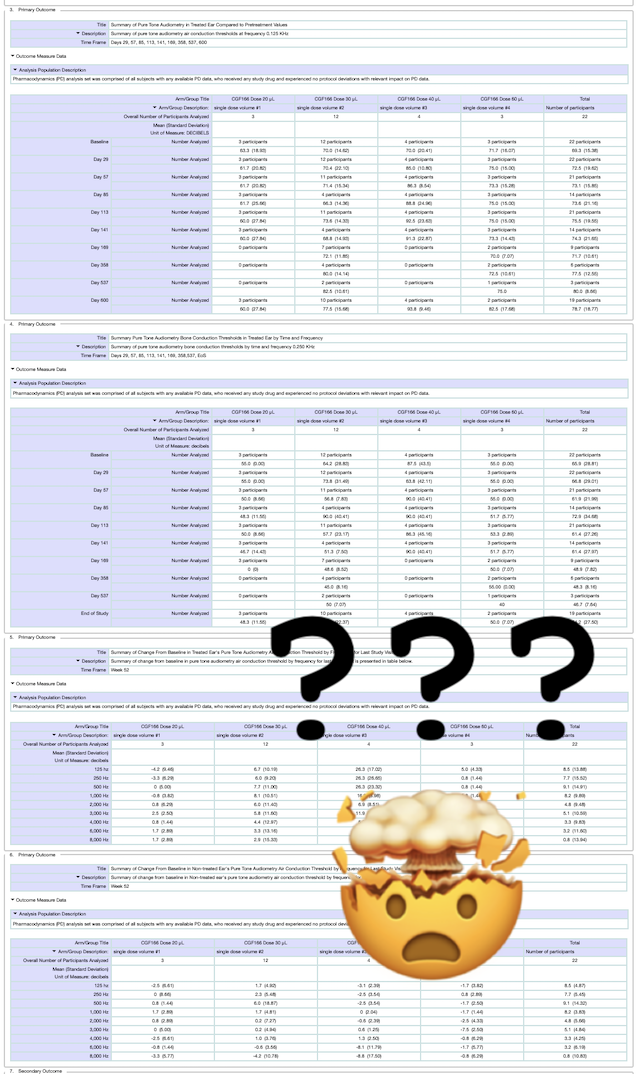 This can be frustrating, but here at Hearing Loss Treatment Report we are currently working on a follow-up post that will provide a clear idea of what these numbers mean for people with hearing loss.
This can be frustrating, but here at Hearing Loss Treatment Report we are currently working on a follow-up post that will provide a clear idea of what these numbers mean for people with hearing loss.
This is a developing story…
I wish we had more for you.
This is all we have, for now.
We will continue to monitor what is happening with CGF166 and post an update as soon as we know more. (The best way to get updates is by subscribing to our email newsletter – which we’ll be sending out very soon!)

For now, we decided to share the link to these “raw” results because we believe in sharing early and sharing often. Information ASAP. Before the official press releases, before company Twitter announcements, before the media outlets, before before before.
And so, for that reason, we didn’t want to sit and wait on this long-awaited study result update just so we could organize it into a neatly-wrapped package with in-depth commentary.
That’s not our style.
“First”
The reason we believe in this post-first-question-later approach is because an increasingly large number of patients, organizations, and industry professionals use this site as a shortcut to the very latest hearing loss treatment updates.
Think of this site like a secret source of some of the fastest, earliest updates in the world of hearing loss drug development. And it’s meant to help inform people in situations like this: where this CGF166 phase I/II results story was missed, ignored, or simply too early (and thus unknown) to the mainstream media.
Which is also why you might want to subscribe to our email updates list… so you can get as-fast-as-we-can-find-them updates on experimental hearing restoration drugs and milestones… plus, sneak previews of what potential “cures” are really around the corner in 2021, 2022, and beyond…
But it’s not for everyone.
Questions? Comments? Corrections? Feedback:
Send an email to michael@urgentresearch.com and say hello.
Prevention of acquired sensorineural hearing loss by in vivo Htra2 gene editing
CATEGORY:
Research
SCREENSHOT:
TITLE:
Prevention of acquired sensorineural hearing loss in mice by in vivo Htra2 gene editing
CONTENT:
Genome Biol. 2021 Mar 22;22(1):86. doi: 10.1186/s13059-021-02311-4.
ABSTRACT
BACKGROUND: Aging, noise, infection, and ototoxic drugs are the major causes of human acquired sensorineural hearing loss, but treatment options are limited. CRISPR/Cas9 technology has tremendous potential to become a new therapeutic modality for acquired non-inherited sensorineural hearing loss. Here, we develop CRISPR/Cas9 strategies to prevent aminoglycoside-induced deafness, a common type of acquired non-inherited sensorineural hearing loss, via disrupting the Htra2 gene in the inner ear which is involved in apoptosis but has not been investigated in cochlear hair cell protection.
RESULTS: The results indicate that adeno-associated virus (AAV)-mediated delivery of CRISPR/SpCas9 system ameliorates neomycin-induced apoptosis, promotes hair cell survival, and significantly improves hearing function in neomycin-treated mice. The protective effect of the AAV-CRISPR/Cas9 system in vivo is sustained up to 8 weeks after neomycin exposure. For more efficient delivery of the whole CRISPR/Cas9 system, we also explore the AAV-CRISPR/SaCas9 system to prevent neomycin-induced deafness. The in vivo editing efficiency of the SaCas9 system is 1.73% on average. We observed significant improvement in auditory brainstem response thresholds in the injected ears compared with the non-injected ears. At 4 weeks after neomycin exposure, the protective effect of the AAV-CRISPR/SaCas9 system is still obvious, with the improvement in auditory brainstem response threshold up to 50 dB at 8 kHz.
CONCLUSIONS: These findings demonstrate the safe and effective prevention of aminoglycoside-induced deafness via Htra2 gene editing and support further development of the CRISPR/Cas9 technology in the treatment of non-inherited hearing loss as well as other non-inherited diseases.
PMID:33752742 | DOI:10.1186/s13059-021-02311-4
SOURCE:
Genome biology
PUBLISHER:
PMID:
pubmed:33752742
ID:
0b58ea4968e09ff10f4e1238c494f316pubmed:33752742
DOI:
10.1186/s13059-021-02311-4
DATE – PUBLISHED:
Tue, 23 Mar 2021 06:00:00 -0400
DATE – DOI:
2021-03-22T09:13:12Z
DATE – ADDED:
03/23/21 07:24AM
LINK – PUBMED:
https://pubmed.ncbi.nlm.nih.gov/33752742/
LINK – DOI:
https://doi.org/10.1186/s13059-021-02311-4
LINK – PUBLISHER:
https://genomebiology.biomedcentral.com/articles/10.1186/s13059-021-02311-4?utm_source=hearinglosstreatmentreport.com
IMAGE:
REFERENCE:
Hearing Loss Treatment Report, Urgent Research, 2021-03-23T11:24:13+00:00, https://www.hearinglosstreatmentreport.com.
SCN11A gene deletion causes sensorineural hearing loss by impairing the ribbon synapses and auditory nerves
CATEGORY:
Research
SCREENSHOT:
TITLE:
SCN11A gene deletion causes sensorineural hearing loss by impairing the ribbon synapses and auditory nerves
CONTENT:
BMC Neurosci. 2021 Mar 22;22(1):18. doi: 10.1186/s12868-021-00613-8.
ABSTRACT
BACKGROUND: The SCN11A gene, encoded Nav1.9 TTX resistant sodium channels, is a main effector in peripheral inflammation related pain in nociceptive neurons. The role of SCN11A gene in the auditory system has not been well characterized. We therefore examined the expression of SCN11A in the murine cochlea, the morphological and physiological features of Nav1.9 knockout (KO) ICR mice.
RESULTS: Nav1.9 expression was found in the primary afferent endings beneath the inner hair cells (IHCs). The relative quantitative expression of Nav1.9 mRNA in modiolus of wild-type (WT) mice remains unchanged from P0 to P60. The number of presynaptic CtBP2 puncta in Nav1.9 KO mice was significantly lower than WT. In addition, the number of SGNs in Nav1.9 KO mice was also less than WT in the basal turn, but not in the apical and middle turns. There was no lesion in the somas and stereocilia of hair cells in Nav1.9 KO mice. Furthermore, Nav1.9 KO mice showed higher and progressive elevated ABR threshold at 16 kHz, and a significant increase in CAP thresholds.
CONCLUSIONS: These data suggest a role of Nav1.9 in regulating the function of ribbon synapses and the auditory nerves. The impairment induced by Nav1.9 gene deletion mimics the characters of cochlear synaptopathy.
PMID:33752606 | DOI:10.1186/s12868-021-00613-8
SOURCE:
BMC neuroscience
PUBLISHER:
PMID:
pubmed:33752606
ID:
0b58ea4968e09ff10f4e1238c494f316pubmed:33752606
DOI:
10.1186/s12868-021-00613-8
DATE – PUBLISHED:
Tue, 23 Mar 2021 06:00:00 -0400
DATE – DOI:
2021-03-22T13:05:35Z
DATE – ADDED:
03/23/21 08:24AM
LINK – PUBMED:
https://pubmed.ncbi.nlm.nih.gov/33752606/
LINK – DOI:
https://doi.org/10.1186/s12868-021-00613-8
LINK – PUBLISHER:
https://bmcneurosci.biomedcentral.com/articles/10.1186/s12868-021-00613-8?utm_source=hearinglosstreatmentreport.com
IMAGE:
REFERENCE:
Hearing Loss Treatment Report, Urgent Research, 2021-03-23T12:24:36+00:00, https://www.hearinglosstreatmentreport.com.
VEGF-A165 restores vascular function to the inner ear and could potentially reverse common forms of hearing loss…
Could improving poor blood supply to the damaged inner ear reverse noise-induced and/or age-related hearing loss?
Yes, according to some fascinating new research from Zhang et al, published yesterday on March 9, 2021, that describes a unique approach to hearing restoration that is backed by “clear-cut evidence”…

Here is a link to a full-text PDF copy of the paper (and all the science-y details):
VEGF-A165 gene therapy ameliorates blood-labyrinth barrier breakdown and hearing loss [PDF]
Sneak preview of the abstract:
When hearing loss is caused by noise or aging, it is often associated with breakdown of the barrier between the cochlea and its blood vessels. Pericytes populate many small vessels in the adult inner ear, however, their role in different forms of hearing loss is largely unknown. Using an inducible and conditional pericyte depletion mouse model, we show that loss of pericytes leads to marked changes in vascular structure, resulting in poor blood circulation and hearing loss. In vitro, using advanced tissue explants from pericyte fluorescence reporter models in combination with exogenous donor pericytes, we show pericytes, signaled by endothelial growth factor isoform A165 (VEGF-A165), vigorously drives new vessel growth in both adult and neonatal mouse inner ear tissue. In vivo, the delivery of an adeno-associated virus serotype 1 (AAV1)-mediated VEGF-A165 viral vector to pericyte depleted animals regenerated lost pericytes, improved blood supply, reduced loss of sensory hair cells, and attenuated hearing loss. These studies provide the first clear-cut evidence that pericytes are critical for adult hearing and can regenerate cochlear vasculature. The restoration of vascular function in the damaged inner ear with AAV1-mediated VEGF-A165 gene therapy is a new strategy for ameliorating vascular associated hearing disorders, including common forms of age-related hearing loss.
SOURCE: Zhang et al. Published March 9, 2021. JCI Insight 2021. https://doi.org/10.1172/jci.insight.143285.
That’s all for now.
The Hearing Loss Treatment Report system will be monitoring the scientific literature and news for any further mentions of VEGF-A165… including any progress it makes toward that first big milestone: human clinical trials.
We don’t have a timeline for you (yet)… but you can expect some follow-up coverage in the coming weeks/months.
Stay tuned.
RE: how to get VEGF-A165 updates
To follow what’s happening with VEGF-A165 (as well as other treatments such as FX-322, OTO-413, PIPE-505), I encourage you to sign up for Hearing Loss Treatment Report email updates. These updates are 100% free… your information is kept private, and… you won’t get any spammy marketing emails or promotional emails. That’s a promise. Instead, you will only receive important (and often exclusive) updates related to up-and-coming hearing loss treatments and scientific breakthroughs.
Questions? Comments? Feedback?
Send an email to michael@urgentresearch.com and speak your mind.
I read every email and try my best to reply (thoughtfully) within 1-2 days.
P.S. Do you like these “exclusive” update posts?
Kindly share them with people you know who might find them interesting.
Advances and challenges in adeno-associated viral inner-ear gene therapy for sensorineural hearing loss
CATEGORY:
Research
SCREENSHOT:
TITLE:
Advances and challenges in adeno-associated viral inner-ear gene therapy for sensorineural hearing loss
CONTENT:
Mol Ther Methods Clin Dev. 2021 Mar 10;21:209-236. doi: 10.1016/j.omtm.2021.03.005. eCollection 2021 Jun 11.
ABSTRACT
There is growing attention and effort focused on treating the root cause of sensorineural hearing loss rather than managing associated secondary characteristic features. With recent substantial advances in understanding sensorineural hearing-loss mechanisms, gene delivery has emerged as a promising strategy for the biological treatment of hearing loss associated with genetic dysfunction. There are several successful and promising proof-of-principle examples of transgene deliveries in animal models; however, there remains substantial further progress to be made in these avenues before realizing their clinical application in humans. Herein, we review different aspects of development, ongoing preclinical studies, and challenges to the clinical transition of transgene delivery of the inner ear toward the restoration of lost auditory and vestibular function.
PMID:33850952 | PMC:PMC8010215 | DOI:10.1016/j.omtm.2021.03.005
SOURCE:
Molecular therapy. Methods & clinical development
PUBLISHER:
PMID:
pubmed:33850952
ID:
0b58ea4968e09ff10f4e1238c494f316pubmed:33850952
DOI:
10.1016/j.omtm.2021.03.005
DATE – PUBLISHED:
Wed, 14 Apr 2021 06:00:00 -0400
DATE – DOI:
2021-03-11T08:48:48Z
DATE – ADDED:
04/14/21 04:52PM
LINK – PUBMED:
https://pubmed.ncbi.nlm.nih.gov/33850952/
LINK – DOI:
https://doi.org/10.1016/j.omtm.2021.03.005
LINK – PUBLISHER:
https://linkinghub.elsevier.com/retrieve/pii/S2329050121000450?utm_source=hearinglosstreatmentreport.com
https://www.cell.com/molecular-therapy-family/methods/fulltext/S2329-0501(21)00045-0
IMAGE:
REFERENCE:
Hearing Loss Treatment Report, Urgent Research, 2021-04-14T20:52:40+00:00, https://www.hearinglosstreatmentreport.com.
VEGF-A165 gene therapy ameliorates blood-labyrinth barrier breakdown and hearing loss
CATEGORY:
Research
SCREENSHOT:
TITLE:
VEGF-A165 gene therapy ameliorates blood-labyrinth barrier breakdown and hearing loss
CONTENT:
JCI Insight. 2021 Mar 9:143285. doi: 10.1172/jci.insight.143285. Online ahead of print.
ABSTRACT
AbstractMillions of people are affected by hearing loss. When hearing loss is caused by noise or aging, it is often associated with breakdown of the barrier between the cochlea and its blood vessels. Pericytes populate many small vessels in the adult inner ear, however, their role in different forms of hearing loss is largely unknown. Using an inducible and conditional pericyte depletion mouse model, we show that loss of pericytes leads to marked changes in vascular structure, resulting in poor blood circulation and hearing loss. In vitro, using advanced tissue explants from pericyte fluorescence reporter models in combination with exogenous donor pericytes, we show pericytes, signaled by endothelial growth factor isoform A165 (VEGF-A165), vigorously drives new vessel growth in both adult and neonatal mouse inner ear tissue. In vivo, the delivery of an adeno-associated virus serotype 1 (AAV1)-mediated VEGF-A165 viral vector to pericyte depleted animals regenerated lost pericytes, improved blood supply, reduced loss of sensory hair cells, and attenuated hearing loss. These studies provide the first clear-cut evidence that pericytes are critical for adult hearing and can regenerate cochlear vasculature. The restoration of vascular function in the damaged inner ear with AAV1-mediated VEGF-A165 gene therapy is a new strategy for ameliorating vascular associated hearing disorders, including common forms of age-related hearing loss.
PMID:33690221 | DOI:10.1172/jci.insight.143285
SOURCE:
JCI insight
PUBLISHER:
PMID:
pubmed:33690221
ID:
0b58ea4968e09ff10f4e1238c494f316pubmed:33690221
DOI:
10.1172/jci.insight.143285
DATE – PUBLISHED:
Wed, 10 Mar 2021 06:00:00 -0500
DATE – DOI:
2021-03-09T17:03:21Z
DATE – ADDED:
03/10/21 10:18PM
LINK – PUBMED:
https://pubmed.ncbi.nlm.nih.gov/33690221/
LINK – DOI:
https://doi.org/10.1172/jci.insight.143285
LINK – PUBLISHER:
http://insight.jci.org/articles/view/143285?utm_source=hearinglosstreatmentreport.com
IMAGE:
REFERENCE:
Hearing Loss Treatment Report, Urgent Research, 2021-03-11T03:18:27+00:00, https://www.hearinglosstreatmentreport.com.
Promise of Optogenetics ‘Sheds Light’ on Hearing Restoration
https://journals.lww.com/thehearingjournal/Fulltext/2021/03000/Promise_of_Optogenetics__Sheds_Light__on_Hearing.1.aspx
Promise of Optogenetics ‘Sheds Light’ on Hearing Restoration
Glantz, Gordon
The Hearing Journal: March 2021 – Volume 74 – Issue 3 – p 20,22,23
doi: 10.1097/01.HJ.0000737552.03771.a4
Decibel Therapeutics to Present at the 44th Annual ARO Conference [DB-OTO gene therapy]
https://ir.decibeltx.com/news-releases/news-release-details/decibel-therapeutics-present-44th-annual-association-research
Decibel Therapeutics to Present at the 44th Annual Association for Research in Otolaryngology (ARO) Conference
Decibel and Catalent Sign Development and Manufacturing Agreement for Dual-Vector Gene Therapy for the Treatment of Hearing Loss
Decibel and Catalent Sign Development and Manufacturing Agreement for Dual-Vector Gene Therapy for the Treatment of Hearing Loss
Stem Cells and Gene Therapy in Progressive Hearing Loss: the State of the Art
CATEGORY:
Research
SCREENSHOT:
TITLE:
Stem Cells and Gene Therapy in Progressive Hearing Loss: the State of the Art
CONTENT:
J Assoc Res Otolaryngol. 2021 Jan 28. doi: 10.1007/s10162-020-00781-0. Online ahead of print.
ABSTRACT
Progressive non-syndromic sensorineural hearing loss (PNSHL) is the most common cause of sensory impairment, affecting more than a third of individuals over the age of 65. PNSHL includes noise-induced hearing loss (NIHL) and inherited forms of deafness, among which is delayed-onset autosomal dominant hearing loss (AD PNSHL). PNSHL is a prime candidate for genetic therapies due to the fact that PNSHL has been studied extensively, and there is a potentially wide window between identification of the disorder and the onset of hearing loss. Several gene therapy strategies exist that show potential for targeting PNSHL, including viral and non-viral approaches, and gene editing versus gene-modulating approaches. To fully explore the potential of these therapy strategies, a faithful in vitro model of the human inner ear is needed. Such models may come from induced pluripotent stem cells (iPSCs). The development of new treatment modalities by combining iPSC modeling with novel and innovative gene therapy approaches will pave the way for future applications leading to improved quality of life for many affected individuals and their families.
PMID:33507440 | DOI:10.1007/s10162-020-00781-0
SOURCE:
Journal of the Association for Research in Otolaryngology : JARO
PUBLISHER:
PMID:
pubmed:33507440
ID:
0b58ea4968e09ff10f4e1238c494f316pubmed:33507440
DOI:
10.1007/s10162-020-00781-0
DATE – PUBLISHED:
Thu, 28 Jan 2021 06:00:00 -0500
DATE – DOI:
2021-01-28T16:56:11Z
DATE – ADDED:
01/28/21 11:57PM
LINK – PUBMED:
https://pubmed.ncbi.nlm.nih.gov/33507440/
LINK – DOI:
https://doi.org/10.1007/s10162-020-00781-0
LINK – PUBLISHER:
http://link.springer.com/10.1007/s10162-020-00781-0
IMAGE:
REFERENCE:
Hearing Loss Treatment Report, Urgent Research, 2021-01-29T04:57:19+00:00, https://www.hearinglosstreatmentreport.com.
Polymorphisms in the FAS gene are associated with susceptibility to noise-induced hearing loss
CATEGORY:
Research
SCREENSHOT:
TITLE:
Polymorphisms in the FAS gene are associated with susceptibility to noise-induced hearing loss
CONTENT:
Environ Sci Pollut Res Int. 2021 Jan 7. doi: 10.1007/s11356-020-12028-9. Online ahead of print.
ABSTRACT
This study investigated the relationship between genetic polymorphisms in the FAS gene and noise-induced hearing loss (NIHL) risk among Chinese workers exposed to occupational noise, and the molecular mechanism of NIHL caused by noise. In this case-control study, 692 NIHL workers and 650 controls were selected for genotyping of four single nucleotide polymorphisms (SNPs) of the FAS gene. Logistic regression was used to calculate the odds ratio (OR) and 95% confidence interval (CI) of the association of these genetic polymorphisms and NIHL. At the same time, a noise-exposed rat model was constructed to further clarify the effect of noise exposure on fas gene expression and the pathogenic mechanism of NIHL. Two polymorphisms, rs1468063 and rs2862833, were associated with NIHL in the case-control study. Individuals with the rs1468063-TT or rs2862833-AA genotypes had decreased NIHL risk (p < 0.01, p = 0.02, respectively). Compared with the control group, the hearing threshold of the case group of rats increased, while serum MDA, urine 8-OHdG, and fas gene expression increased, but let-7e expression decreased. Genetic polymorphisms in the FAS gene are related to the risk of NIHL in the Chinese population. Noise can cause a large amount of reactive oxygen species (ROS) in the cochlea tissue and blood, which lead to oxidative stress, lipid peroxidation, and DNA damage, further activating the FAS gene, and ultimately leading to hearing loss. PMID:33411277 | DOI:10.1007/s11356-020-12028-9 SOURCE: Environmental science and pollution research international PUBLISHER: PMID: pubmed:33411277 ID: 0b58ea4968e09ff10f4e1238c494f316pubmed:33411277 DOI: 10.1007/s11356-020-12028-9 DATE - PUBLISHED: Thu, 07 Jan 2021 06:00:00 -0500 DATE - DOI: 2021-01-07T08:06:27Z DATE - ADDED: 01/07/21 06:43PM LINK - PUBMED: https://pubmed.ncbi.nlm.nih.gov/33411277/ LINK - DOI: https://doi.org/10.1007/s11356-020-12028-9 LINK - PUBLISHER: http://link.springer.com/10.1007/s11356-020-12028-9 IMAGE: REFERENCE: Hearing Loss Treatment Report, Urgent Research, 2021-01-07T23:43:34+00:00, https://www.hearinglosstreatmentreport.com.
Promising targets for deafness drug discovery
Promising targets for deafness drug discovery
Single and dual vector gene therapy with AAV9-PHP.B rescues hearing in Tmc1 mutant mice
CATEGORY:
Research
TITLE:
Single and dual vector gene therapy with AAV9-PHP.B rescues hearing in Tmc1 mutant mice
DESCRIPTION:
AAV-mediated gene therapy is a promising approach for treating genetic hearing loss. Replacement or editing of the Tmc1 gene, encoding hair cell mechanosensory ion channels, is effective for hearing restoration in mice with some limitations. Efficient rescue of outer hair cell function, as well as lack of hearing recovery with later stage treatment, remain issues to be solved. Exogenous genes delivered with the AAV9-PHP.B capsid via the utricle transduce both inner and outer hair cells of the…
CONTENT:
Mol Ther. 2020 Nov 16:S1525-0016(20)30614-6. doi: 10.1016/j.ymthe.2020.11.016. Online ahead of print.
ABSTRACT
AAV-mediated gene therapy is a promising approach for treating genetic hearing loss. Replacement or editing of the Tmc1 gene, encoding hair cell mechanosensory ion channels, is effective for hearing restoration in mice with some limitations. Efficient rescue of outer hair cell function, as well as lack of hearing recovery with later stage treatment, remain issues to be solved. Exogenous genes delivered with the AAV9-PHP.B capsid via the utricle transduce both inner and outer hair cells of the mouse cochlea with high efficacy. Here we demonstrate that AAV9-PHP.B gene therapy can promote hair cell survival and successfully rescues hearing in three distinct mouse models of hearing loss. Tmc1 replacement with AAV9-PHP.B in a Tmc1 knockout mouse rescues hearing and promotes hair cell survival with equal efficacy in inner and outer hair cells. The same treatment in a recessive Tmc1 hearing loss model, Baringo, partially recovers hearing even with later stage treatment. Finally, dual delivery of SpCas9 and gRNA in separate AAV9-PHP.B vectors selectively disrupts a dominant Tmc1 allele and preserves hearing in Beethoven mice, a model of dominant, progressive hearing loss. Tmc1-targeted gene therapies using single or dual AAV9-PHP.B vectors offer potent and versatile approaches for treating dominant and recessive deafness.
PMID:33212302 | DOI:10.1016/j.ymthe.2020.11.016
SOURCE:
Molecular therapy : the journal of the American Society of Gene Therapy
DATE – PUBLISHED:
16 Nov 2020
DATE – ADDED:
Thu, 19 Nov 2020 06:00:00 -0500
DATE – FOUND:
11/20/20 05:29AM
PUBMED ID:
pubmed:33212302
DOI:
10.1016/j.ymthe.2020.11.016
PUBMED LINK:
https://pubmed.ncbi.nlm.nih.gov/33212302/
DOI LINK:
https://doi.org/10.1016/j.ymthe.2020.11.016
PUBLISHER LINK:
https://linkinghub.elsevier.com/retrieve/pii/S1525001620306146
Decibel Therapeutics Announces Exclusive Licensing Agreements for Hearing Loss Gene Therapy Technology
https://www.biospace.com/article/releases/decibel-therapeutics-announces-exclusive-licensing-agreements-for-hearing-loss-gene-therapy-technology/
Decibel Therapeutics Announces Exclusive Licensing Agreements for Hearing Loss Gene Therapy Technology
Loss of RAD6B induces degeneration of the cochlea in mice
https://www.sciencedirect.com/science/article/abs/pii/S0006291X2031562X?via%3Dihub
https://pubmed.ncbi.nlm.nih.gov/32868078/
Loss of RAD6B induces degeneration of the cochlea in mice
Yangping Ma 1, Yanfeng Song 1, Rong Shen 1, Panpan Li 1, Han Ding 1, Zhao Guo 1, Xiangwen Liu 1, Degui Wang 2
Affiliations expand
PMID: 32868078 DOI: 10.1016/j.bbrc.2020.08.017
Abstract
Presbycusis is a form of age-related hearing loss (AHL). Many studies have shown that the degeneration of various structures in the cochlea of the inner ear is related to AHL, and DNA damage is an important factor leading to the above process. As an E2 ubiquitin-conjugated enzyme, RAD6B plays an important role in DNA damage repair (DDR) through histone ubiquitination. However, the molecular mechanism is still unclear. In this study, we investigated the role of RAD6B in the morphological changes and DDR mechanisms in aging-related degeneration of the cochlea of mice. We observed that the hair cells, stria vascularis and spiral ganglion in the cochlea of the RAD6B knockout mice showed significant degenerative changes and abnormal expression of proteins associated with DDR mechanisms compared with those of the littermate wild-type mice. In conclusion, our results suggest that the deletion of RAD6B may lead to abnormalities in DDR, thereby accelerating the degeneration of various structures in the cochlea and senescence and apoptosis of cochlea cells.
Keywords: Cochlea; DNA damage; Degeneration; RAD6B; Ubiquitin.
Recent Advancements in Understanding the Role of Epigenetics in the Auditory System
https://www.sciencedirect.com/science/article/abs/pii/S037811192030665X?via%3Dihub
Review Gene
2020 Jul 29;144996. doi: 10.1016/j.gene.2020.144996. Online ahead of print.
Recent Advancements in Understanding the Role of Epigenetics in the Auditory System
Rahul Mittal 1, Nicole Bencie 1, George Liu 1, Nicolas Eshraghi 1, Eric Nisenbaum 1, Susan H Blanton 2, Denise Yan 1, Jeenu Mittal 1, Christine T Dinh 1, Juan I Young 3, Feng Gong 4, Xue Zhong Liu 5
Affiliations expand
PMID: 32738421 DOI: 10.1016/j.gene.2020.144996
Restoring hearing in mice
https://www.nature.com/articles/s41684-020-0605-2
In Brief
Published: 23 July 2020
GENE EDITING
Restoring hearing in mice
Alexandra Le Bras
Lab Animal volume 49, page220(2020)Cite this article
18 Accesses
Metricsdetails
Yeh, W-H. et al. Sci. Transl. Med. 12, eaay9101 (2020)
Genetic defects are a major cause of hearing loss (HL) in newborns. No curative treatments are available for genetic HL, but gene therapy-based strategies that replace an absent gene product or silence a pathological allele have shown promising results in mouse models.
A study describes a new base-editing approach aimed at correcting a point mutation in Tmc1 that causes deafness in Baringo mice. Adeno-associated virus (AAV) delivery of a cytosine base editor and guide RNA into the inner ears of Baringo mice at postnatal day 1 successfully corrected the Tmc1 mutation and partially rescued auditory function, thereby demonstrating the potential of base editing as a treatment for HL caused by recessive loss-of-function point mutations.
Inner Ear Gene Therapies Take Off: Current Promises and Future Challenges
https://www.mdpi.com/2077-0383/9/7/2309/htm
https://pubmed.ncbi.nlm.nih.gov/32708116/
Inner Ear Gene Therapies Take Off: Current Promises and Future Challenges
by Sedigheh Delmaghani *OrcID andAziz El-Amraoui *OrcID
Progressive Sensory Disorders, Pathophysiology and Therapy Unit, Institut Pasteur, Institut de l’Audition, INSERM-UMRS1120, Sorbonne Université, 63 rue de Charenton, 75012 Paris, France
Authors to whom correspondence should be addressed.
J. Clin. Med. 2020, 9(7), 2309; https://doi.org/10.3390/jcm9072309
Received: 27 June 2020 / Revised: 13 July 2020 / Accepted: 15 July 2020 / Published: 21 July 2020
Gene therapy development in hearing research in China
https://www.nature.com/articles/s41434-020-0177-1
https://www.ncbi.nlm.nih.gov/pubmed/32681137?dopt=Abstract
Related Articles
Gene therapy development in hearing research in China.
Gene Ther. 2020 Jul 17;:
Authors: Zhang Z, Wang J, Li C, Xue W, Xing Y, Liu F
Abstract
Sensorineural hearing loss, the most common form of hearing impairment, is mainly attributable to genetic mutations or acquired factors, such as aging, noise exposure, and ototoxic drugs. In the field of gene therapy, advances in genetic and physiological studies and profound increases in knowledge regarding the underlying mechanisms have yielded great progress in terms of restoring the auditory function in animal models of deafness. Nonetheless, many challenges associated with the translation from basic research to clinical therapies remain to be overcome before a total restoration of auditory function can be expected. In recent years, Chinese research teams have promoted various developmental efforts in this field, including gene sequencing to identify additional potential loci that cause deafness, studies to elucidate the underlying molecular mechanisms, and research to optimize vectors and delivery routes. In this review, we summarize the state of the field and focus mainly on the progress of gene therapy in animal model studies and the optimization of therapeutic strategies in China.
PMID: 32681137 [PubMed – as supplied by publisher]
Generation of inner ear hair cells by direct lineage conversion of primary somatic cells
https://elifesciences.org/articles/55249
https://www.ncbi.nlm.nih.gov/pubmed/32602462?dopt=Abstract
Related Articles
Generation of inner ear hair cells by direct lineage conversion of primary somatic cells.
Elife. 2020 Jun 30;9:
Authors: Menendez L, Trecek T, Gopalakrishnan S, Tao L, Markowitz AL, Yu HV, Wang X, Llamas J, Huang C, Lee J, Kalluri R, Ichida J, Segil N
Abstract
The mechanoreceptive sensory hair cells in the inner ear are selectively vulnerable to numerous genetic and environmental insults. In mammals, hair cells lack regenerative capacity, and their death leads to permanent hearing loss and vestibular dysfunction. Their paucity and inaccessibility has limited the search for otoprotective and regenerative strategies. Growing hair cells in vitro would provide a route to overcome this experimental bottleneck. We report a combination of four transcription factors (Six1, Atoh1, Pou4f3, and Gfi1) that can convert mouse embryonic fibroblasts, adult tail-tip fibroblasts and postnatal supporting cells into induced hair cell-like cells (iHCs). iHCs exhibit hair cell-like morphology, transcriptomic and epigenetic profiles, electrophysiological properties, mechanosensory channel expression, and vulnerability to ototoxin in a high-content phenotypic screening system. Thus, direct reprogramming provides a platform to identify causes and treatments for hair cell loss, and may help identify future gene therapy approaches for restoring hearing.
PMID: 32602462 [PubMed – in process]
Hair Cell Transduction Efficiency of Single- and Dual-AAV Serotypes in Adult Murine Cochleae: findings broaden the application of cochlear gene therapy targeting hair cells [PDF]
https://www.cell.com/molecular-therapy-family/methods/pdf/S2329-0501(20)30094-2.pdf?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS2329050120300942%3Fshowall%3Dtrue
https://www.ncbi.nlm.nih.gov/pubmed/32518805?dopt=Abstract
Related Articles
Hair Cell Transduction Efficiency of Single- and Dual-AAV Serotypes in Adult Murine Cochleae.
Mol Ther Methods Clin Dev. 2020 Jun 12;17:1167-1177
Authors: Omichi R, Yoshimura H, Shibata SB, Vandenberghe LH, Smith RJH
Abstract
Gene delivery is a key component for the treatment of genetic hearing loss. To date, a myriad of adeno-associated virus (AAV) serotypes and surgical approaches have been employed to deliver transgenes to cochlear hair cells, but the efficacy of dual transduction remains unclear. Herein, we investigated cellular tropism of single injections of AAV serotype 1 (AAV1), AAV2, AAV8, AAV9, and Anc80L65, and quantitated dual-vector co-transduction rates following co-injection of AAV2 and AAV9 vectors in adult murine cochlea. We used the combined round window membrane and canal fenestration (RWM+CF) injection technique for vector delivery. Single AAV2 injections were most robust and transduced 96.7% ± 1.1% of inner hair cells (IHCs) and 83.9% ± 2.0% of outer hair cells (OHCs) throughout the cochlea without causing hearing impairment or hair cell loss. Dual AAV2 injection co-transduced 96.9% ± 1.7% of IHCs and 65.6% ± 8.95% of OHCs. Together, RWM+CF-injected single or dual AAV2 provides the highest auditory hair cell transduction efficiency of the AAV serotypes we studied. These findings broaden the application of cochlear gene therapy targeting hair cells.
PMID: 32518805 [PubMed]
In vivo base editing restores sensory transduction and transiently improves auditory function in a mouse model of recessive deafness
https://stm.sciencemag.org/content/12/546/eaay9101
https://www.ncbi.nlm.nih.gov/pubmed/32493795?dopt=Abstract
Related Articles
In vivo base editing restores sensory transduction and transiently improves auditory function in a mouse model of recessive deafness.
Sci Transl Med. 2020 Jun 03;12(546):
Authors: Yeh WH, Shubina-Oleinik O, Levy JM, Pan B, Newby GA, Wornow M, Burt R, Chen JC, Holt JR, Liu DR
Abstract
Most genetic diseases arise from recessive point mutations that require correction, rather than disruption, of the pathogenic allele to benefit patients. Base editing has the potential to directly repair point mutations and provide therapeutic restoration of gene function. Mutations of transmembrane channel-like 1 gene (TMC1) can cause dominant or recessive deafness. We developed a base editing strategy to treat Baringo mice, which carry a recessive, loss-of-function point mutation (c.A545G; resulting in the substitution p.Y182C) in Tmc1 that causes deafness. Tmc1 encodes a protein that forms mechanosensitive ion channels in sensory hair cells of the inner ear and is required for normal auditory function. We found that sensory hair cells of Baringo mice have a complete loss of auditory sensory transduction. To repair the mutation, we tested several optimized cytosine base editors (CBEmax variants) and guide RNAs in Baringo mouse embryonic fibroblasts. We packaged the most promising CBE, derived from an activation-induced cytidine deaminase (AID), into dual adeno-associated viruses (AAVs) using a split-intein delivery system. The dual AID-CBEmax AAVs were injected into the inner ears of Baringo mice at postnatal day 1. Injected mice showed up to 51% reversion of the Tmc1 c.A545G point mutation to wild-type sequence (c.A545A) in Tmc1 transcripts. Repair of Tmc1 in vivo restored inner hair cell sensory transduction and hair cell morphology and transiently rescued low-frequency hearing 4 weeks after injection. These findings provide a foundation for a potential one-time treatment for recessive hearing loss and support further development of base editing to correct pathogenic point mutations.
PMID: 32493795 [PubMed – in process]
Viral gene delivery of Yap5SA in the postnatal inner ear sensory epithelia in vivo drives cell cycle reentry after hair cell loss: new strategies to induce sensory cell regeneration
https://www.pnas.org/content/117/24/13552
Organ of Corti size is governed by Yap/Tead-mediated progenitor self-renewal
Role of Yap/Tead transcription factor complex in maintaining inner ear progenitors during development: new strategies to induce sensory cell regeneration
View ORCID ProfileKsenia Gnedeva, View ORCID ProfileXizi Wang, Melissa M. McGovern, Matthew Barton, Litao Tao, Talon Trecek, View ORCID ProfileTanner O. Monroe, Juan Llamas, Welly Makmura, James F. Martin, Andrew K. Groves, Mark Warchol, and View ORCID ProfileNeil Segil
PNAS June 16, 2020 117 (24) 13552-13561; first published June 1, 2020 https://doi.org/10.1073/pnas.2000175117
Edited by Marianne E. Bronner, California Institute of Technology, Pasadena, CA, and approved April 21, 2020 (received for review January 6, 2020)
Significance
While Yap/Tead signaling is well known to influence tissue growth and organ size during development, the molecular outputs of the pathway are tissue- and context-dependent and remain poorly understood. Our work expands the mechanistic understanding of how Yap/Tead signaling controls the precise number of progenitor cells that will be laid down within the developing inner ear to ultimately regulate the final size and function of the sensory organs. We also provide evidence that restoration of hearing and vestibular function may be amenable to YAP-mediated regeneration. Our data show that reactivation of Yap/Tead signaling after hair cell loss induces a proliferative response in vivo—a process thought to be permanently repressed in the mammalian inner ear.
Abstract
Precise control of organ growth and patterning is executed through a balanced regulation of progenitor self-renewal and differentiation. In the auditory sensory epithelium—the organ of Corti—progenitor cells exit the cell cycle in a coordinated wave between E12.5 and E14.5 before the initiation of sensory receptor cell differentiation, making it a unique system for studying the molecular mechanisms controlling the switch between proliferation and differentiation. Here we identify the Yap/Tead complex as a key regulator of the self-renewal gene network in organ of Corti progenitor cells. We show that Tead transcription factors bind directly to the putative regulatory elements of many stemness- and cell cycle-related genes. We also show that the Tead coactivator protein, Yap, is degraded specifically in the Sox2-positive domain of the cochlear duct, resulting in down-regulation of Tead gene targets. Further, conditional loss of the Yap gene in the inner ear results in the formation of significantly smaller auditory and vestibular sensory epithelia, while conditional overexpression of a constitutively active version of Yap, Yap5SA, is sufficient to prevent cell cycle exit and to prolong sensory tissue growth. We also show that viral gene delivery of Yap5SA in the postnatal inner ear sensory epithelia in vivo drives cell cycle reentry after hair cell loss. Taken together, these data highlight the key role of the Yap/Tead transcription factor complex in maintaining inner ear progenitors during development, and suggest new strategies to induce sensory cell regeneration.
Recent development of AAV-based gene therapies for inner ear disorders
https://www.nature.com/articles/s41434-020-0155-7
https://www.ncbi.nlm.nih.gov/pubmed/32424232?dopt=Abstract
Related Articles
Recent development of AAV-based gene therapies for inner ear disorders.
Gene Ther. 2020 May 18;:
Authors: Lan Y, Tao Y, Wang Y, Ke J, Yang Q, Liu X, Su B, Wu Y, Lin CP, Zhong G
Abstract
Gene therapy for auditory diseases is gradually maturing. Recent progress in gene therapy treatments for genetic and acquired hearing loss has demonstrated the feasibility in animal models. However, a number of hurdles, such as lack of safe viral vector with high efficiency and specificity, robust deafness large animal models, translating animal studies to clinic etc., still remain to be solved. It is necessary to overcome these challenges in order to effectively recover auditory function in human patients. Here, we review the progress made in our group, especially our efforts to make more effective and cell type-specific viral vectors for targeting cochlea cells.
PMID: 32424232 [PubMed – as supplied by publisher]
Decibel Therapeutics, a clinical-stage biotechnology company developing novel gene therapeutics for restoration of hearing loss, to present at the 23rd Annual Meeting of the American Society of Gene & Cell Therapy (ASGCT)
https://www.businesswire.com/news/home/20200511005122/en/
Decibel Therapeutics to Present at the 23rd Annual Meeting of the American Society of Gene & Cell Therapy (ASGCT)
Akouos to Present Data from Inner Ear Gene Therapy Platform at 23rd ASGCT Annual Meeting
https://www.businesswire.com/news/home/20200511005866/en/Akouos-Present-Data-Ear-Gene-Therapy-Platform
Akouos to Present Data from Inner Ear Gene Therapy Platform at 23rd ASGCT Annual Meeting
Gene therapy for hair cell regeneration: Review and new data
https://www.sciencedirect.com/science/article/abs/pii/S0378595519304964?via%3Dihub
https://www.ncbi.nlm.nih.gov/pubmed/32563621?dopt=Abstract
Gene therapy for hair cell regeneration: Review and new data.
Hear Res. 2020 May 05;:107981
Authors: Shibata SB, West MB, Du X, Iwasa Y, Raphael Y, Kopke RD
Abstract
Hair cells (HCs) in the cochlea are responsible for transducing mechanical sound energy into neural impulses which lead to the perception of sound. Loss of these sensory cells is the most common cause of sensorineural hearing loss, and spontaneous HC regeneration does not occur in mature mammals. Among the future potential treatment modalities is gene therapy, which is defined as the administration of either DNAs or RNAs as active pharmaceutical ingredients for inducing a clinically-beneficial response. Gene therapy is being envisioned and evaluated as a potential tool for addressing a number of human inner ear disorders. This paper is a hybrid Review and Research Paper, including unpublished data and a review of HC regeneration studies in live animal models. Current gene therapeutic approaches for replacing lost HC populations have been aimed at converting supporting cells surviving within the neuro-epithelium to new HCs by inducing upregulation of bHLH transcription factors such as Atoh1 or reciprocal silencing of Notch signaling with siRNAs, to tip the balance of transcriptional regulation toward a HC fate. Development of one or more of these techniques may yield a path to effective restoration of inner ear form and function. This review also describes other approaches and molecular targets that may prove efficacious and provides perspectives on future clinical challenges and opportunities for gene therapy to become a valuable weapon for the long-anticipated realization of this regenerative treatment.
PMID: 32563621 [PubMed – as supplied by publisher]
Role of microRNA in inner ear stem cells and related research progress: solving the medical problem of inner ear hair cell regeneration
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7218733/
https://www.ncbi.nlm.nih.gov/pubmed/32419976?dopt=Abstract
Related Articles
Role of microRNA in inner ear stem cells and related research progress.
Am J Stem Cells. 2020;9(2):16-24
Authors: Wu X, Zou S, Wu F, He Z, Kong W
Abstract
Deafness is one of the major global health problems that seriously affects the quality of human life. At present, there are no successful treatments for deafness caused by cochlear hair cell (HC) damage. The irreversibility of mammalian hearing impairment is that the inner ear’s sensory epithelium cannot repair lost hair cells and neurons through spontaneous regeneration. The goal of stem cell therapy for sensorineural hearing loss is to reconstruct the damaged inner ear structure and achieve functional repair. microRNA (miRNA), as a class of highly conserved endogenous non-coding small RNAs, plays an important role in the development of cochlea and HCs. miRNA also participates in the regulation of stem cell proliferation and differentiation, and plays an important role in the process of regeneration of inner ear HCs, miRNA has a broad application prospect of clinical treatment of hearing loss, which is conducive to solving the medical problem of inner ear HC regeneration.
PMID: 32419976 [PubMed]
Making the Case for Research on Disease-Modifying Treatments to Tackle Post-lingual Progressive Sensorineural Hearing Loss
https://www.frontiersin.org/articles/10.3389/fneur.2020.00290/full
Front. Neurol., 21 April 2020 | https://doi.org/10.3389/fneur.2020.00290
Making the Case for Research on Disease-Modifying Treatments to Tackle Post-lingual Progressive Sensorineural Hearing Loss
Vincent Van Rompaey1,2*
1Department of Otorhinolaryngology and Head & Neck Surgery, Antwerp University Hospital, Edegem, Belgium
2Department of Translational Neurosciences, Faculty of Medicine and Health Sciences, University of Antwerp, Antwerp, Belgium
Diagnostic and Therapeutic Applications of Genomic Medicine in Sensorineural Hearing Loss
https://www.sciencedirect.com/science/article/abs/pii/S0378111920303462?via%3Dihub
https://www.ncbi.nlm.nih.gov/pubmed/32304785?dopt=Abstract
Related Articles
Diagnostic and Therapeutic Applications of Genomic Medicine in Progressive, Late-Onset, Nonsyndromic Sensorineural Hearing Loss.
Gene. 2020 Apr 15;:144677
Authors: Jimenez JE, Nourbakhsh A, Colbert B, Mittal R, Yan D, Green CL, Nisenbaum E, Liu G, Bencie N, Rudman J, Blanton SH, Zhong Liu X
Abstract
The progressive, late-onset, nonsyndromic, sensorineural hearing loss (PNSHL) is the most common cause of sensory impairment globally, with presbycusis affecting greater than a third of individuals over the age of 65. The etiology underlying PNSHL include presbycusis, noise-induced hearing loss, drug ototoxicity, and delayed-onset autosomal dominant hearing loss (AD PNSHL). The objective of this article is to discuss the potential diagnostic and therapeutic applications of genomic medicine in PNSHL. Genomic factors contribute greatly to PNSHL. The heritability of presbycusis ranges from 25 to 75%. Current therapies for PNSHL range from sound amplification to cochlear implantation (CI). PNSHL is an excellent candidate for genomic medicine approaches as it is common, has well-described pathophysiology, has a wide time window for treatment, and is amenable to local gene therapy by currently utilized procedural approaches. AD PNSHL is especially suited to genomic medicine approaches that can disrupt the expression of an aberrant protein product. Gene therapy is emerging as a potential therapeutic strategy for the treatment of PNSHL. Viral gene delivery approaches have demonstrated promising results in human clinical trials for two inherited causes of blindness and are being used for PNSHL in animal models and a human trial. Non-viral gene therapy approaches are useful in situations where a transient biologic effect is needed or for delivery of genome editing reagents (such as CRISPR/Cas9) into the inner ear. Many gene therapy modalities that have proven efficacious in animal trials have potential to delay or prevent PNSHL in humans. The development of new treatment modalities for PNSHL will lead to improved quality of life of many affected individuals and their families.
PMID: 32304785 [PubMed – as supplied by publisher]
Neurotrophin gene therapy to promote survival of spiral ganglion neurons after deafness
https://www.sciencedirect.com/science/article/abs/pii/S0378595519304563?via%3Dihub
https://www.ncbi.nlm.nih.gov/pubmed/32331858?dopt=Abstract
Related Articles
Neurotrophin gene therapy to promote survival of spiral ganglion neurons after deafness.
Hear Res. 2020 Apr 05;:107955
Authors: Leake PA, Akil O, Lang H
Abstract
Hearing impairment is a major health and economic concern worldwide. Currently, the cochlear implant (CI) is the standard of care for remediation of severe to profound hearing loss, and in general, contemporary CIs are highly successful. But there is great variability in outcomes among individuals, especially in children, with many CI users deriving much less or even marginal benefit. Much of this variability is related to differences in auditory nerve survival, and there has been substantial interest in recent years in exploring potential therapies to improve survival of the cochlear spiral ganglion neurons (SGN) after deafness. Preclinical studies using osmotic pumps and other approaches in deafened animal models to deliver neurotrophic factors (NTs) directly to the cochlea have shown promising results, especially with Brain-Derived Neurotrophic Factor (BDNF). More recent studies have focused on the use of NT gene therapy to force expression of NTs by target cells within the cochlea. This could provide the means for a one-time treatment to promote long-term NT expression and improve neural survival after deafness. This review summarizes the evidence for the efficacy of exogenous NTs in preventing SGN degeneration after hearing loss and reviews the animal research to date suggesting that NT gene therapy can elicit long-term NT expression in the cochlea, resulting in significantly improved SGN and radial nerve fiber survival after deafness. In addition, we discuss NT gene therapy in other non-auditory applications and consider some of the remaining issues with regard to selecting optimal vectors, timing of treatment, and place/method of delivery, etc. that must be resolved prior to considering clinical application.
PMID: 32331858 [PubMed – as supplied by publisher]
Akouos Raises $105 Million in Funding to Advance First-In-Human Clinical Studies of AK-OTOF, a Potential Gene Therapy for Sensorineural Hearing Loss
https://www.businesswire.com/news/home/20200303005317/en/Akouos-Closes-105-Million-Series-Financing
Precision Genetic Medicine Company Akouos Secures $105 Million In Funding
Akouos Raises $105 Million in Funding to Advance First-In-Human Clinical Studies of AK-OTOF, a Potential Gene Therapy for Sensorineural Hearing Loss
rAAV-Mediated Cochlear Gene Therapy: Prospects and Challenges for Clinical Application
https://www.mdpi.com/2077-0383/9/2/589
https://www.ncbi.nlm.nih.gov/pubmed/32098144?dopt=Abstract
Related Articles
rAAV-Mediated Cochlear Gene Therapy: Prospects and Challenges for Clinical Application.
J Clin Med. 2020 Feb 21;9(2):
Authors: Blanc F, Mondain M, Bemelmans AP, Affortit C, Puel JL, Wang J
Abstract
Over the last decade, pioneering molecular gene therapy for inner-ear disorders have achieved experimental hearing improvements after a single local or systemic injection of adeno-associated, virus-derived vectors (rAAV for recombinant AAV) encoding an extra copy of a normal gene, or ribozymes used to modify a genome. These results hold promise for treating congenital or later-onset hearing loss resulting from monogenic disorders with gene therapy approaches in patients. In this review, we summarize the current state of rAAV-mediated inner-ear gene therapies including the choice of vectors and delivery routes, and discuss the prospects and obstacles for the future development of efficient clinical rAAV-mediated cochlear gene medicine therapy.
PMID: 32098144 [PubMed]
AAV9-PHP.B vector drives gene expression in inner ear hair cells and could serve as a gene therapy vehicle for treatment of inner ear dysfunction in humans
https://www.ncbi.nlm.nih.gov/pubmed/31980281?dopt=Abstract
https://www.sciencedirect.com/science/article/pii/S0378595519305246?via%3Dihub
Lastly, we report here that alternate promoters, Syn and Gfap, can be used in combination with AAV9-PHP.B capsids to drive selective expression in subsets of cochlear cell types, such as neurons and supporting cells. We conclude that the AAV9-PHP.B capsid is a powerful vector for driving exogenous gene expression in pre-clinical mouse models of hearing and balance disorders and may be well-suited for further development and perhaps translation to clinical application as a gene therapy vehicle for treatment of inner ear dysfunction in humans.
Related Articles
Efficient viral transduction in mouse inner ear hair cells with utricle injection and AAV9-PHP.B.
Hear Res. 2020 Jan 13;:107882
Authors: Lee J, Nist-Lund C, Solanes P, Goldberg H, Wu J, Pan B, Schneider BL, Holt JR
Abstract
Viral delivery of exogenous coding sequences into the inner ear has the potential for therapeutic benefit for patients suffering genetic or acquired hearing loss. To devise improved strategies for viral delivery, we investigated two injection techniques, round window membrane injection or a novel utricle injection method, for their ability to safely and efficiently transduce sensory hair cells and neurons of the mouse inner ear. In addition, we evaluated three synthetic AAV vectors (Anc80L65, AAV9-PHP.B, AAV2.7m8) encoding enhanced green fluorescent protein (eGFP) and three promoters (Cmv, Synapsin, Gfap) for their ability to transduce and drive expression in desired cell types. We found the utricle injection method with AAV9-PHP.B and a Cmv promoter was the most efficient combination for driving robust eGFP expression in both inner and outer hair cells. We found eGFP expression levels rose over 3-5 days post-injection, a viral dose of 1.5 × 109 gc yielded half maximal eGFP expression and that the utricle injection method yielded transduced hair cells even when delivered as late as postnatal day 16. Sensory transduction and auditory thresholds were unaltered in injected mice relative to uninjected wild-type controls. Vestibular end organs were also transduced without affecting balance behavior. The Synapsin promoter and the Gfap promoter drove strong eGFP expression in inner ear neurons and supporting cells, respectively. We conclude the AAV9-PHP.B vector and the utricle injection method are well-suited for delivery of exogenous gene constructs into inner ears of mouse models of auditory and vestibular dysfunction.
PMID: 31980281 [PubMed – as supplied by publisher]
Targeting the Inner Ear: New startups are taking aim at delivering drugs to help treat hearing loss
Targeting the Inner Ear
New startups are taking aim at delivering drugs to help treat hearing loss
Genetic therapies for hearing loss: Accomplishments and remaining challenges
https://link.springer.com/article/10.1007%2Fs11306-019-1595-1
https://www.ncbi.nlm.nih.gov/pubmed/31586696?dopt=Abstract
Genetic therapies for hearing loss: Accomplishments and remaining challenges.
Neurosci Lett. 2019 Oct 03;:134527
Authors: Taiber S, Avraham KB
Abstract
More than 15 years have passed since the official completion of the Human Genome Project. Predominantly due to this project, over one hundred genes have now been linked to hearing loss. Although major advancements have been made in the understanding of underlying pathologies in deafness as a consequence of these gene discoveries, biological treatments for these conditions are still not available and current treatments rely on amplification or prosthetics. A promising approach for developing treatments for genetic hearing loss is the most simplistic one, that of gene therapy. Gene therapy would intuitively be ideal for these conditions since it is directed at the very source of the problem. Recent achievements in this field in laboratory models spike hope and optimism among scientists, patients, and industry, and suggest that this approach can mature into clinical trials in the coming years. Here we review the existing literature and discuss the different aspects of developing gene therapy for genetic hearing loss.
PMID: 31586696 [PubMed – as supplied by publisher]
AGTC and Otonomy Announce Strategic Collaboration to Develop and Commercialize Gene Therapy for Congenital Hearing Loss
https://finance.yahoo.com/news/agtc-otonomy-announce-strategic-collaboration-113000562.html
AGTC and Otonomy Announce Strategic Collaboration to Develop and Commercialize Gene Therapy for Congenital Hearing Loss
A new study has identified 44 genes linked to age-related hearing loss giving a much clearer understanding of how the condition develops and potential treatments
https://www.sciencedaily.com/releases/2019/09/190926073350.htm
Akouos Discloses Lead Program, AK-OTOF, a Potential Gene Therapy for Sensorineural Hearing Loss
https://www.businesswire.com/news/home/20190912005094/en/Akouos-Discloses-Lead-Program-AK-OTOF-Potential-Gene
Akouos Discloses Lead Program, AK-OTOF, a Potential Gene Therapy for Sensorineural Hearing Loss
Gene Therapy for Human Sensorineural Hearing Loss
https://www.frontiersin.org/articles/10.3389/fncel.2019.00323/abstract
Gene Therapy for Human Sensorineural Hearing Loss
Gene therapy for sensorineural hearing loss (SNHL)
https://academic.oup.com/hmg/advance-article-abstract/doi/10.1093/hmg/ddz129/5520925?redirectedFrom=fulltext
https://www.ncbi.nlm.nih.gov/pubmed/31227837?dopt=Abstract
Related Articles
Gene therapy for hearing loss.
Hum Mol Genet. 2019 Jun 22;:
Authors: Omichi R, Shibata SB, Morton CC, Smith RJH
Abstract
Sensorineural hearing loss (SNHL) is the most common sensory disorder. Its underlying etiologies include a broad spectrum of genetic and environmental factors that can lead to hearing loss that is congenital or late onset, stable or progressive, drug-related, noise-induced, age-related, traumatic or post-infectious. Habilitation options typically focus on amplification using wearable or implantable devices, however exciting new gene-therapy based strategies to restore and prevent SNHL are actively under investigation. Recent proof-of-principle studies demonstrate the potential therapeutic potential of molecular agents delivered to the inner ear to ameliorate different types of SNHL. Correcting or preventing underlying genetic forms of hearing loss is poised to become a reality. Herein, we review molecular therapies for hearing loss such as gene replacement, antisense oligonucleotides, RNA interference and CRISPR-based gene editing. We discuss delivery methods, techniques and viral vectors employed for inner ear gene therapy, and the advancements in this field that are paving the way for basic science research discoveries to transition to clinical trials.
PMID: 31227837 [PubMed – as supplied by publisher]
Gene Editing Based Hearing Impairment Research and Therapeutics
https://www.ncbi.nlm.nih.gov/pubmed/31195050?dopt=Abstract
Gene Editing Based Hearing Impairment Research and Therapeutics.
Neurosci Lett. 2019 Jun 10;:134326
Authors: Kang W, Sun Z, Zhao X, Wang X, Tao Y, Wu H
Abstract
Hearing impairment affects 1 in 500 newborns worldwide and nearly one out of three people over the age of 65 (WHO, 2019). Hereditary hearing loss is the most common type of congenital deafness; genetic factors also affect deafness susceptibility. Gene therapies may preserve or restore natural sound perception, and have rescued deafness in multiple hereditary murine models. CRISPR-Cas9 and base editors (BEs) are newly developed gene-editing technologies that can facilitate gene studies in the inner ear and provide therapeutic approaches for hearing impairment. Here, we present recent applications of gene editing in the inner ear.
PMID: 31195050 [PubMed – as supplied by publisher]
Intratympanically Delivered Steroids Impact Thousands More Inner Ear Genes Than Systemic Delivery
https://journals.sagepub.com/doi/abs/10.1177/0003489419837562?journalCode=aora
https://www.ncbi.nlm.nih.gov/pubmed/31092042?dopt=Abstract
Related Articles
Intratympanically Delivered Steroids Impact Thousands More Inner Ear Genes Than Systemic Delivery.
Ann Otol Rhinol Laryngol. 2019 Jun;128(6_suppl):134S-138S
Authors: Trune DR, Shives KD, Hausman F, Kempton JB, MacArthur CJ, Choi D
Abstract
OBJECTIVES: Glucocorticoids are given for sensorineural hearing loss, but little is known of their molecular impact on the inner ear. Furthermore, in spite of claims of improved hearing recovery with intratympanic delivery of steroids, no studies have actually documented the inner ear molecular functions that are enhanced with this delivery method.
METHODS: To assess steroid-driven processes in the inner ear, gene chip analyses were conducted on mice treated systemically with the glucocorticoids prednisolone or dexamethasone or the mineralocorticoid aldosterone. Other mice were given the same steroids intratympanically. Inner ears were harvested at 6 hours and processed on the Affymetrix 430 2.0 Gene Chip for expression of its 34 000 genes. Results were statistically analyzed for up or down expression of each gene against control (untreated) mice.
RESULTS: Analyses showed approximately 17 500 genes are normally expressed in the inner ear and steroids alter expression of 55% to 82% of these. Dexamethasone changed expression of 9424 (53.9%) inner ear genes following systemic injection but 14 899 ear genes (85%) if given intratympanically. A similar pattern was seen with prednisolone, as 7560 genes were impacted by oral delivery and 11 164 genes (63.8%) when given intratympanically. The mineralocorticoid aldosterone changed expression of only 268 inner ear genes if given orally, but this increased to 10 124 genes (57.9%) if injected intratympanically. Furthermore, the glucocorticoids given actually impacted more inner ear genes via the mineralocorticoid receptor than the glucocorticoid receptor.
CONCLUSIONS: Thousands of inner ear genes were affected by steroids, and this number increased significantly if steroids were delivered intratympanically. Also, the impact of glucocorticoids on inner ear mineralocorticoid functions is more substantial than previously known. Thus, the application of therapeutic steroids for hearing loss needs to be reassessed in light of their more comprehensive impact on inner ear genes. Furthermore, simply ascribing the efficacy of steroids to immunosuppression no longer appears to be warranted.
PMID: 31092042 [PubMed – in process]
Novartis begins recruiting patients with hearing loss for Phase 2 (Part C) of inner ear gene therapy [CGF166] trial
https://clinicaltrials.gov/ct2/show/NCT02132130?type=Intr
New molecular therapies for the treatment of hearing loss
https://www.sciencedirect.com/science/article/pii/S0163725819300774?via%3Dihub
https://www.ncbi.nlm.nih.gov/pubmed/31075354?dopt=Abstract
New molecular therapies for the treatment of hearing loss.
Pharmacol Ther. 2019 May 07;:
Authors: Ma Y, Wise AK, Shepherd RK, Richardson RT
Abstract
An estimated 466 million people suffer from hearing loss worldwide. Sensorineural hearing loss is characterized by degeneration of key structures of the sensory pathway in the cochlea such as the sensory hair cells, the primary auditory neurons and their synaptic connection to the hair cells – the ribbon synapse. Various strategies to protect or regenerate these sensory cells and structures are the subject of intensive research. Yet despite recent advances in our understandings of the capacity of the cochlea for repair and regeneration there are currently no pharmacological or biological interventions for hearing loss. Current research focusses on localized cochlear drug, gene and cell-based therapies. One of the more promising drug-based therapies is based on neurotrophic factors for the repair of the ribbon synapse after noise exposure, as well as preventing loss of primary auditory neurons and regrowth of the auditory neuron fibers after severe hearing loss. Drug therapy delivery technologies are being employed to address the specific needs of neurotrophin and other therapies for hearing loss that include the need for high doses, long-term delivery, localised or cell-specific targeting and techniques for their safe and efficacious delivery to the cochlea. Novel biomaterials are enabling high payloads of drugs to be administered to the cochlea with subsequent slow-release properties that are proving to be beneficial for treating hearing loss. In parallel, new gene therapy technologies are addressing the need for cell specificity and high efficacy for the treatment of both genetic and acquired hearing loss with promising reports of hearing recovery. Some biomaterials and cell therapies are being used in conjunction with the cochlear implant ensuring therapeutic benefit to the primary neurons during electrical stimulation. This review will introduce the auditory system, hearing loss and the potential for repair and regeneration in the cochlea. Drug delivery to the cochlea will then be reviewed, with a focus on new biomaterials, gene therapy technologies, cell therapy and the use of the cochlear implant as a vehicle for drug delivery. With the current pre-clinical research effort into therapies for hearing loss, including clinical trials for gene therapy, the future for the treatment for hearing loss is looking bright.
PMID: 31075354 [PubMed – as supplied by publisher]
Polymorphism in GRHL2 gene may contribute to noise-induced hearing loss susceptibility
https://www.sciencedirect.com/science/article/pii/S1808869418304397?via%3Dihub
https://www.ncbi.nlm.nih.gov/pubmed/30853467?dopt=Abstract
Related Articles
Polymorphism in GRHL2 gene may contribute to noise-induced hearing loss susceptibility: a meta-analysis.
Braz J Otorhinolaryngol. 2020 May – Jun;86(3):370-375
Authors: Li X, Zhu Z, Li W, Wei L, Zhao B, Hao Z
Abstract
INSTRUCTION: Noise-induced hearing loss is a leading occupational disease caused by gene-environment interaction. The Grainy Like 2, GRHL2, is a candidate gene. In this regard, many studies have evaluated the association between GRHL2 and noise-induced hearing loss, although the results are ambiguous and conflicting.
OBJECTIVE: The purpose of this study was to identify a precise estimation of the association between rs3735715 polymorphism in GRHL2 gene and susceptibility of noise-induced hearing loss.
METHODS: A comprehensive search was performed to collect data up to July 8, 2018. Finally, 4 eligible articles were included in this meta-analysis comprising 2410 subjects. The pooled odds ratios with 95% confidence intervals were used to evaluate the strength of the association.
RESULTS: Significant association was found in the overall population in the dominant model (GA/AA vs. GG, odds ratio=0.707, 95% confidence interval=0.594-0.841) and allele model (G allele vs. A allele, odds ratio=1.189, 95% confidence interval=1.062-1.333). When stratified by source of the subjects, we also found association between rs3735715 and noise-induced hearing loss risk in the dominant model (GA/AA vs. GG, odds ratio=0.634, 95% confidence interval=0.514-0.783) and allele model (G allele vs. A allele, odds ratio=1.206, 95% confidence interval=1.054-1.379).
CONCLUSION: Rs3735715 polymorphism in GRHL2 gene may influence the susceptibility of noise-induced hearing loss. Additional large, well-designed and functional studies are needed to confirm this association in different populations.
PMID: 30853467 [PubMed – indexed for MEDLINE]
Dual AAV-mediated gene therapy restores hearing in a DFNB9 mouse model
https://www.pnas.org/content/116/10/4496
https://www.ncbi.nlm.nih.gov/pubmed/30782832?dopt=Abstract
Related Articles
Dual AAV-mediated gene therapy restores hearing in a DFNB9 mouse model.
Proc Natl Acad Sci U S A. 2019 03 05;116(10):4496-4501
Authors: Akil O, Dyka F, Calvet C, Emptoz A, Lahlou G, Nouaille S, Boutet de Monvel J, Hardelin JP, Hauswirth WW, Avan P, Petit C, Safieddine S, Lustig LR
Abstract
Autosomal recessive genetic forms (DFNB) account for most cases of profound congenital deafness. Adeno-associated virus (AAV)-based gene therapy is a promising therapeutic option, but is limited by a potentially short therapeutic window and the constrained packaging capacity of the vector. We focus here on the otoferlin gene underlying DFNB9, one of the most frequent genetic forms of congenital deafness. We adopted a dual AAV approach using two different recombinant vectors, one containing the 5′ and the other the 3′ portions of otoferlin cDNA, which exceed the packaging capacity of the AAV when combined. A single delivery of the vector pair into the mature cochlea of Otof -/- mutant mice reconstituted the otoferlin cDNA coding sequence through recombination of the 5′ and 3′ cDNAs, leading to the durable restoration of otoferlin expression in transduced cells and a reversal of the deafness phenotype, raising hopes for future gene therapy trials in DFNB9 patients.
PMID: 30782832 [PubMed – indexed for MEDLINE]
AAV2.7m8 is a powerful viral vector for inner ear gene therapy
https://www.nature.com/articles/s41467-018-08243-1
https://www.ncbi.nlm.nih.gov/pubmed/30683875