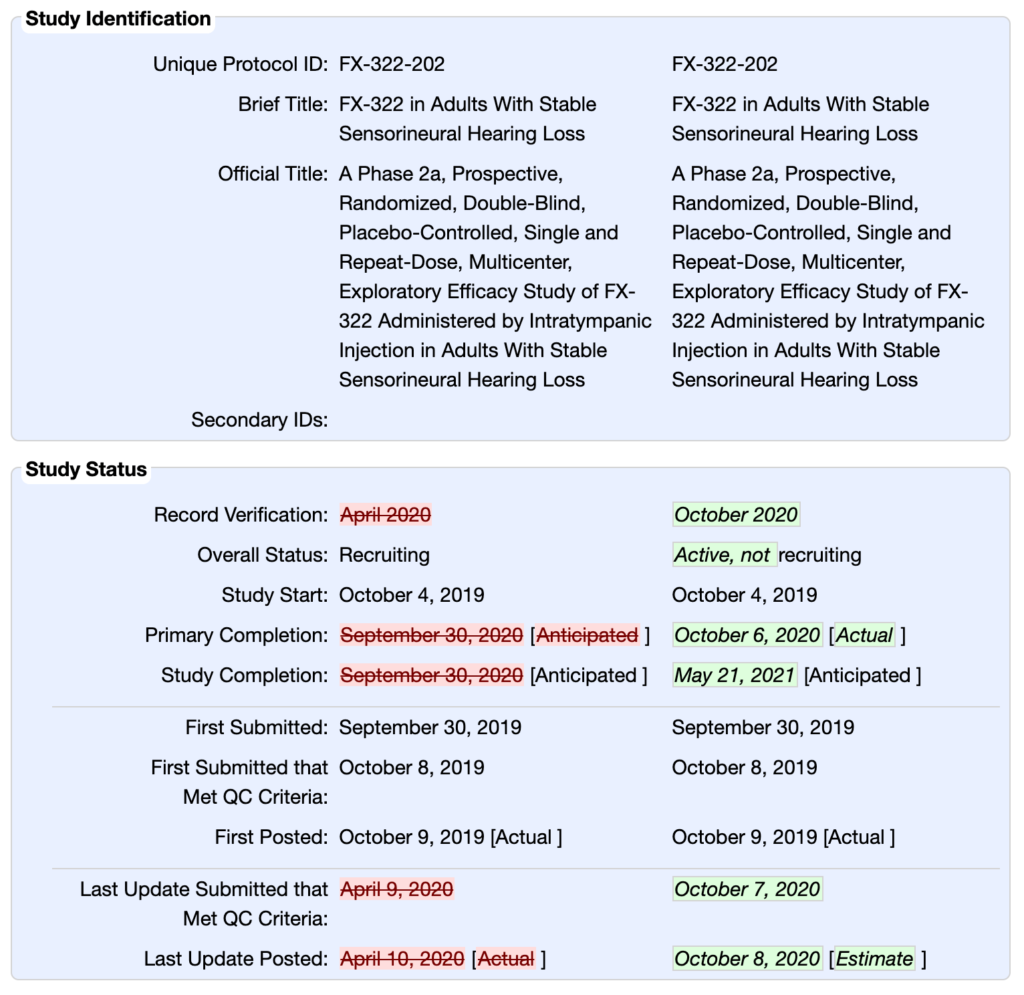
CATEGORY:
Research
SCREENSHOT:
TITLE:
Protective effects of vitamins/antioxidants on occupational noise-induced hearing loss: A systematic review
CONTENT:
J Occup Health. 2021 Jan;63(1):e12217. doi: 10.1002/1348-9585.12217.
ABSTRACT
OBJECTIVES: Occupational noise-induced hearing loss (NIHL) due to industrial, military, and other job -related noise exposure can cause harmful health issues to occupied workers, but may also be potentially preventable. Vitamins/antioxidant have been studied as therapeutic strategies to prevent and/or delay the risks of human diseases as well as NIHL .So, this study was conducted to systematically review the protective effects of vitamins/antioxidants on occupational NIHL.
METHODS: Online databases including PubMed/Medline, Scopus, Web of Science, EMBASE, Science Direct, and Google Scholar were systematically searched up to 12 January 2021. Based on 6336 potentially relevant records identified through the initial search in the databases, 12 full-text publications were retrieved, one of which can be viewed as two separate trials, because it has studied the effects of two different antioxidants (ginseng and NAC) on NIHL, separately.
RESULTS: A review of the studies shows that vitamin B12, folic acid, and N-acetylcysteine (NAC) have a considerable protective effect on NIHL. However, these protective effects are not yet specified in different frequencies. The findings regarding the protective effects of other antioxidants are inconsistent in this field.
CONCLUSION: Vitamin B12, folic acid, and NAC may have a protective effect as an antioxidant on reducing occupational hearing loss. For a conclusive evidence of vitamin/antioxidant protective therapies, future studies with precise criteria for noise exposure and similar outcome parameters are required.
PMID:33788342 | DOI:10.1002/1348-9585.12217
SOURCE:
Journal of occupational health
PUBLISHER:
PMID:
pubmed:33788342
ID:
0b58ea4968e09ff10f4e1238c494f316pubmed:33788342
DOI:
10.1002/1348-9585.12217
DATE – PUBLISHED:
Wed, 31 Mar 2021 06:00:00 -0400
DATE – DOI:
2021-03-31T14:18:59Z
DATE – ADDED:
03/31/21 07:53PM
LINK – PUBMED:
https://pubmed.ncbi.nlm.nih.gov/33788342/
LINK – DOI:
https://doi.org/10.1002/1348-9585.12217
LINK – PUBLISHER:
https://onlinelibrary.wiley.com/doi/10.1002/1348-9585.12217?utm_source=hearinglosstreatmentreport.com
IMAGE:
REFERENCE:
Hearing Loss Treatment Report, Urgent Research, 2021-03-31T23:53:37+00:00, https://www.hearinglosstreatmentreport.com.