CATEGORY:
Research
SCREENSHOT:
TITLE:
Surface electrical stimulation of the auditory cortex preserves efferent medial olivocochlear neurons and reduces cochlear traits of age-related hearing loss
CONTENT:
Hear Res. 2024 Apr 12;447:109008. doi: 10.1016/j.heares.2024.109008. Online ahead of print.
ABSTRACT
The auditory cortex is the source of descending connections providing contextual feedback for auditory signal processing at almost all levels of the lemniscal auditory pathway. Such feedback is essential for cognitive processing. It is likely that corticofugal pathways are degraded with aging, becoming important players in age-related hearing loss and, by extension, in cognitive decline. We are testing the hypothesis that surface, epidural stimulation of the auditory cortex during aging may regulate the activity of corticofugal pathways, resulting in modulation of central and peripheral traits of auditory aging. Increased auditory thresholds during ongoing age-related hearing loss in the rat are attenuated after two weeks of epidural stimulation with direct current applied to the surface of the auditory cortex for two weeks in alternate days (Fernández del Campo et al., 2024). Here we report that the same cortical electrical stimulation protocol induces structural and cytochemical changes in the aging cochlea and auditory brainstem, which may underlie recovery of age-degraded auditory sensitivity. Specifically, we found that in 18 month-old rats after two weeks of cortical electrical stimulation there is, relative to age-matched non-stimulated rats: a) a larger number of choline acetyltransferase immunoreactive neuronal cell body profiles in the ventral nucleus of the trapezoid body, originating the medial olivocochlear system.; b) a reduction of age-related dystrophic changes in the stria vascularis; c) diminished immunoreactivity for the pro-inflammatory cytokine TNFα in the stria vascularis and spiral ligament. d) diminished immunoreactivity for Iba1 and changes in the morphology of Iba1 immunoreactive cells in the lateral wall, suggesting reduced activation of macrophage/microglia; d) Increased immunoreactivity levels for calretinin in spiral ganglion neurons, suggesting excitability modulation by corticofugal stimulation. Altogether, these findings support that non-invasive neuromodulation of the auditory cortex during aging preserves the cochlear efferent system and ameliorates cochlear aging traits, including stria vascularis dystrophy, dysregulated inflammation and altered excitability in primary auditory neurons.
PMID:38636186 | DOI:10.1016/j.heares.2024.109008
SOURCE:
Hearing research
PUBLISHER:
PMID:
pubmed:38636186
ID:
0b58ea4968e09ff10f4e1238c494f316pubmed:38636186
DOI:
10.1016/j.heares.2024.109008
DATE – PUBLISHED:
Thu, 18 Apr 2024 06:00:00 -0400
DATE – DOI:
2024-04-12T19:54:49Z
DATE – ADDED:
04/19/24 12:40AM
LINK – PUBMED:
https://pubmed.ncbi.nlm.nih.gov/38636186/
LINK – DOI:
https://doi.org/10.1016/j.heares.2024.109008
LINK – PUBLISHER:
https://linkinghub.elsevier.com/retrieve/pii/S0378595524000613?utm_source=hearinglosstreatmentreport.com
IMAGE:
REFERENCE:
Hearing Loss Treatment Report, Urgent Research, 2024-04-19T04:40:15+00:00, https://www.hearinglosstreatmentreport.com.








 What does that mean?
What does that mean?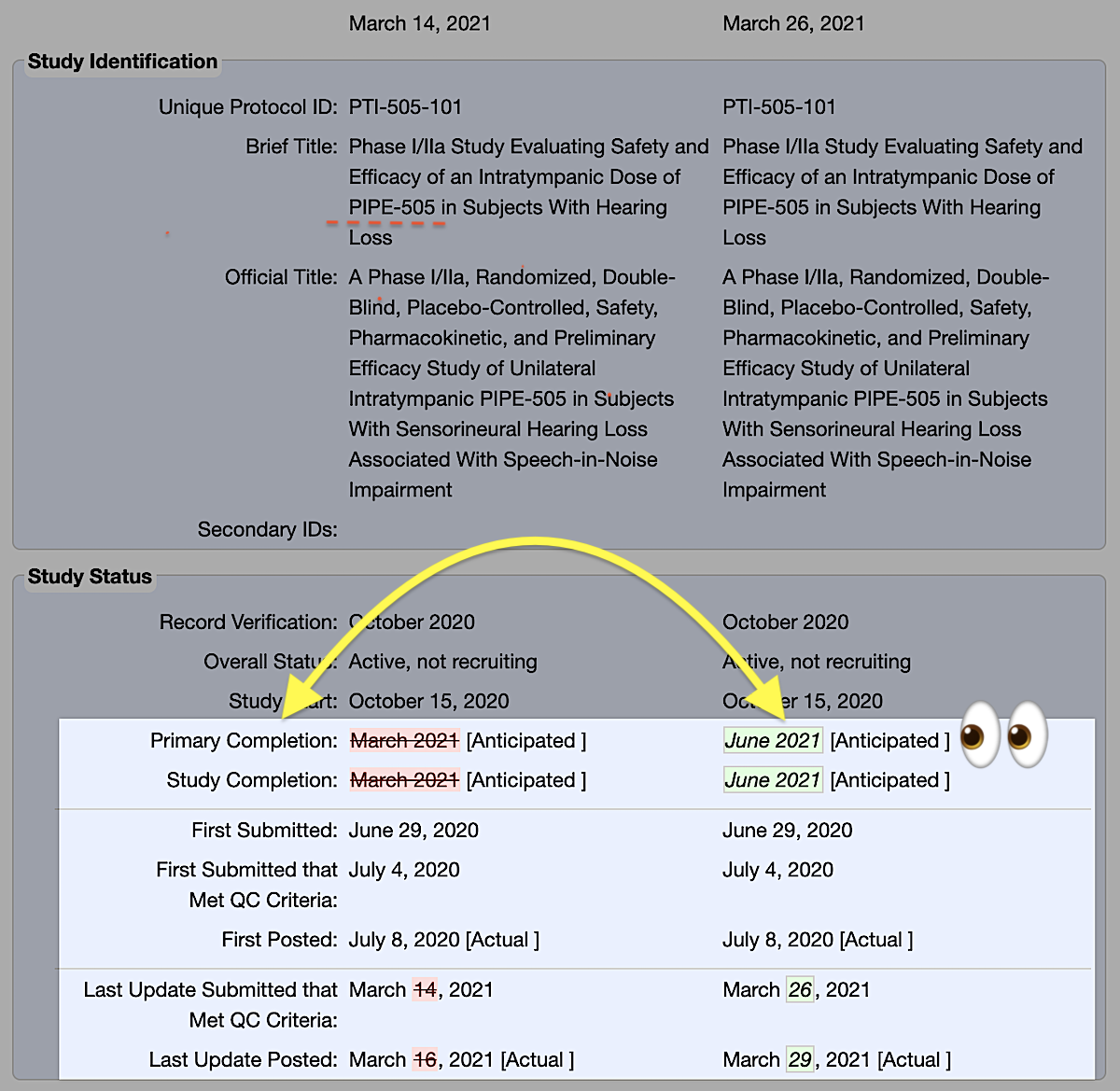
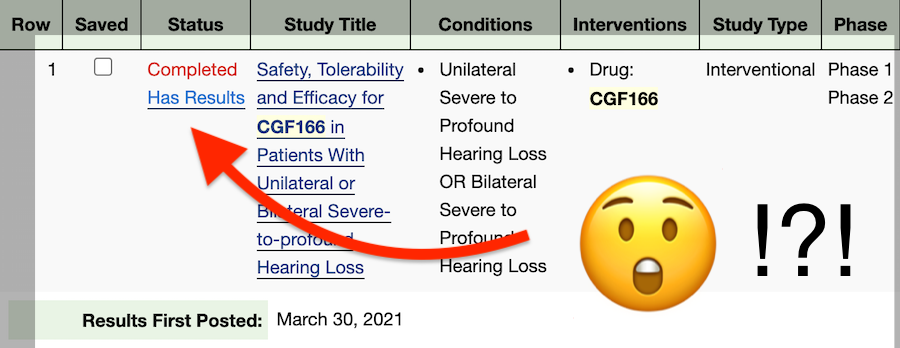
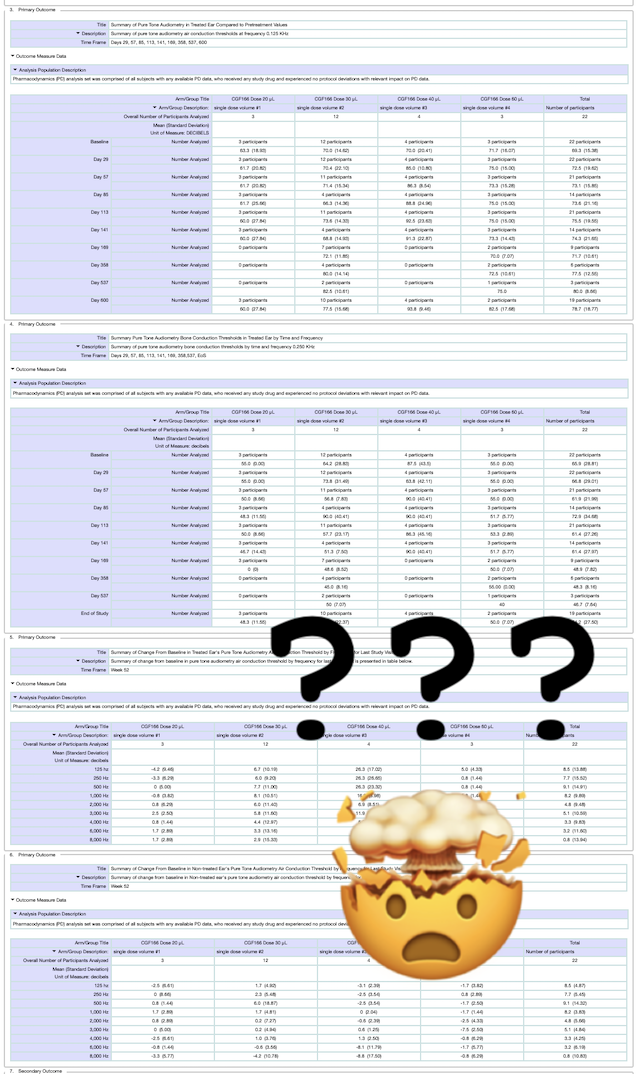 This can be frustrating, but here at Hearing Loss Treatment Report we are currently working on a follow-up post that will provide a clear idea of what these numbers mean for people with hearing loss.
This can be frustrating, but here at Hearing Loss Treatment Report we are currently working on a follow-up post that will provide a clear idea of what these numbers mean for people with hearing loss.