https://clinicaltrials.gov/ct2/show/NCT04462198
PIPE-505
Estimated PIPE-505 Phase 2a Study Completion Date: June 12, 2021
Short but sweet update:
We now have an estimated completion date for the PIPE-505 phase 2a clinical trial: June 12, 2021.
Details below:
- The PIPE-505 study record was updated on March 29, 2021.
- The study completion date was changed from March 2021 [Anticipated] to June 2021 [Anticipated].
- The June 12, 2021 date [Estimated] was calculated using information found within the study record and its history of changes, including: a) the submission date on which the Study Status was changed from “Recruiting” to “Active, not recruiting” and, b) the maximum follow-up Time Frame (“3 months after drug administration”) as detailed in the Outcome Measures section.
- For a more conservative completion date, replace the last submitted update to Study Status date with the more recent last submitted update date of March 26, 2021. This gives us June 24, 2021.
- Taken together, we are looking at a range of June 12 – June 24, with June 12th as the one to mark on your calendar.
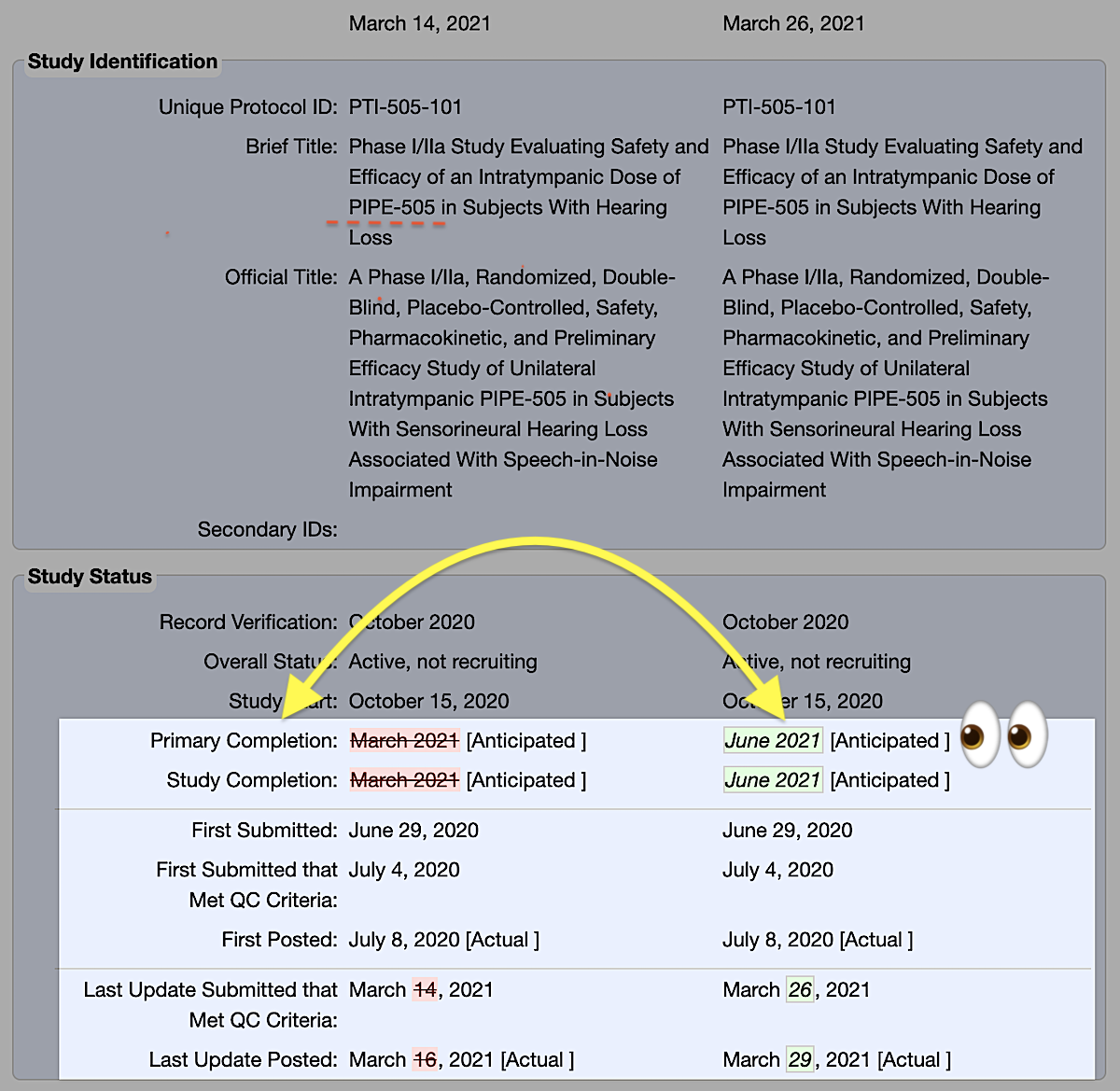
Source: History of Changes for Study: NCT04462198 (Phase I/IIa Study Evaluating Safety and Efficacy of an Intratympanic Dose of PIPE-505 in Subjects With Hearing Loss)
That’s all for now.
We will probably have the next PIPE-505 update for you before June 15th (and/or whenever Pipeline Therapeutics makes an announcement).
For ongoing PIPE-505 updates and news of other upcoming hearing loss treatments, sign up for the free email newsletter. No spam, no promotional emails, privacy respected. Between 1-3 emails per week (but only when something interesting is happening).
Phase I/IIa Study Evaluating Safety and Efficacy of an Intratympanic Dose of PIPE-505 in Subjects With Hearing Loss
CATEGORY:
Clinical Trials
TITLE:
Phase I/IIa Study Evaluating Safety and Efficacy of an Intratympanic Dose of PIPE-505 in Subjects With Hearing Loss
INTERVENTION/TREATMENT:
PHASE:
DESCRIPTION:
Condition : Sensorineural Hearing Loss
Interventions : Drug: PIPE-505; Drug: Diluent alone
Sponsor : Pipeline Therapeutics, Inc.
Recruiting
ID:
NCT04462198
STATUS:
DATE – FIRST POSTED:
Wed, 08 Jul 2020 12:00:00 EDT
DATE – LAST UPDATE POSTED:
10/14/20 08:47PM
DATE – RETRIEVED:
10/14/20 08:47PM
LINK – STUDY HISTORY:
https://clinicaltrials.gov/ct2/history/NCT04462198
LINK – STUDY RECORD:
https://clinicaltrials.gov/ct2/show/NCT04462198
PIPE-505 Clinical Trial Update: Pipeline adds 4 new study locations across the United States…
As anticipated, Pipeline Therapeutics has added additional clinical trial sites to its phase 1/2a study of PIPE-505 for hearing loss.
The official study record (NCT04462198), updated on August 6, 2020, now includes five study locations across the U.S., four of which are now recruiting participants:
- Boca Raton, Florida, United States, 33487 – added July 17 – Recruiting
- Winston-Salem, North Carolina, United States, 27103 – added July 27 – Recruiting
- New Albany, Indiana, United States, 47150 – added August 1 – Recruiting
- Louisville, Kentucky, United States, 40220 – added August 1 – Recruiting
- Kansas City, Kansas, United States, 66160 – added August 4 – Not yet recruiting
This post will be updated to include any further changes to the study record. For email updates (which will be starting soon), join the announcement list while it is still open.
Pipeline Therapeutics Initiates Phase 1/2a Clinical Trial of PIPE-505 in Sensorineural Hearing Loss – Topline results expected in early 2021…
https://www.pipelinetherapeutics.com/news/pr_07-23-2020.php
Pipeline Therapeutics Initiates Phase 1/2a Clinical Trial of PIPE-505 in Sensorineural Hearing Loss
PIPE-505 is the first small molecule developed specifically for the treatment of sensorineural hearing loss (SNHL) associated with speech-in-noise impairment
Topline results expected in early 2021
SAN DIEGO, July 23, 2020 – Pipeline Therapeutics, a biopharmaceutical company focused on the development and commercialization of first-in-class small molecules for neuroregeneration, today announced the initiation of a Phase 1/2a trial of the company’s lead product candidate, PIPE-505, a small molecule gamma secretase inhibitor (GSI), in sensorineural hearing loss (SNHL) associated with hearing speech in noisy environments.
UPDATE: Pipeline Therapeutics Begins Recruiting Patients for Phase 1/2a Study of PIPE-505 for SNHL
This is a follow-up post to the previous PIPE-505 clinical trial update from July 8 where we explained how “patient recruitment could begin any day now.”
Today, less than two weeks later, that forecast proved accurate: the PIPE-505 trial is now RECRUITING, according to the official study record (NCT04462198), which had its Recruitment Status updated yesterday.
Here is an excerpt from that ClinicalTrials.gov record:
STUDY TITLE: A Phase I/IIa, Randomized, Double-Blind, Placebo-Controlled, Safety, Pharmacokinetic, and Preliminary Efficacy Study of Unilateral Intratympanic PIPE-505 in Subjects With Sensorineural Hearing Loss Associated With Speech-in-Noise Impairment
Recruitment Status: Recruiting
First Posted: July 8, 2020
Last Update Posted: July 20, 2020DESCRIPTION: This is a randomized-controlled, double-blind study of PIPE-505 or placebo given as an injection one time in subjects with sensorineural hearing loss associated with speech-in-noise difficulty. Visits to the clinic will occur at baseline, dosing, and days 1, 7, 14, 30, 60 and 90 after treatment. Safety will be assessed by periodic measurement of vital signs, ear examination, electrocardiogram (ECG), blood laboratory analyses and occurrence of adverse events (AE). Efficacy will be assessed by periodic audiometry and other audiological tests.
ClinicalTrials.gov Identifier: NCT04462198
Where is the PIPE-505 study located?
As of July 21, 2020, there is only one recruitment location (Florida) for the trial. But it is common for studies like this one to expand and add more locations across the country and sometimes internationally, too. We expect more recruitment sites to be added in the next 4-6 weeks, although we do not have an exact list of those locations… yet…
UPDATE: August 29, 2020 – A second recruitment location (North Carolina) has been added to the list. (More to come?)
More updates to follow for PIPE-505 and other up-and-coming hearing loss treatments. Keep an eye on the front page of this website for updates, or get email updates (it’s an upcoming once-weekly email newsletter – no spam, no third parties, privacy respected).
People on the email list will get first access to new versions of this website that track more research from more sources…
Questions? Comments? Corrections? Collaborations? Send an email to michael@urgentresearch.com and say hello.
Pipeline Therapeutics Initiates Phase 1/2 Clinical Trial of PIPE-505 for Sensorineural Hearing Loss
The study titled, Phase I/IIa Study Evaluating Safety and Efficacy of an Intratympanic Dose of PIPE-505 in Subjects With Hearing Loss, was added to the official ClinicalTrials.gov databased on July 8, 2020.
The recruitment status of the clinical trial is currently set to “Not yet recruiting.” However, that could change very soon, considering a) the follow-up period for trial participants after the initial drug administration lasts 3 months, and b) the anticipated primary completion date of the study is four months away, in November 2020.
These details suggest that patient recruitment could begin any day now and – assuming there are no changes to the study’s current schedule – probably no later than 4-6 weeks from now.
UPDATE (current as of July 21, 2020): the PIPE-505 study is now recruiting patients… see this follow-up post for details or visit the official study page using the link below:
https://clinicaltrials.gov/ct2/show/NCT04462198
Here is further reading about PIPE-505, a small molecule gamma secretase inhibitor (GSI) that will be delivered to select trial participants as a one-time intratympanic injection:
PIPE-505 is the first small molecule developed specifically for the treatment of sensorineural hearing loss (SNHL) associated with cochlear synaptopathy. The therapeutic focus, regeneration of the cochlear synapse, should augment signal-to-noise processing and manifest as improved speech-in-noise comprehension, a chief auditory complaint and unmet need of patients with SNHL.
Source: https://www.pipelinetherapeutics.com/science/synaptogenesis.html
Pipeline Therapeutics Completes $30 Million Series B Financing – Proceeds to advance PIPE-505 in hearing loss
https://www.businesswire.com/news/home/20191218005137/en/Pipeline-Therapeutics-Completes-30-Million-Series-Financing
Pipeline Therapeutics’ lead candidate, PIPE-505, is entering Phase 1b/2a testing to treat mild-to-moderate sensorineural hearing loss (SNHL) associated with cochlear synaptopathy. PIPE-505 is uniquely positioned to treat this patient population as it can have an impact on both audibility and speech intelligibility, addressing two separate pathologies associated with hearing loss.
Pipeline Therapeutics Completes $30 Million Series B Financing – Proceeds to advance PIPE-505 in hearing loss
Discovery of PIPE-505, a small molecule therapeutic for the treatment of sensorineural hearing loss (SNHL) associated with cochlear synaptopathy [PDF]
https://www.pipelinetherapeutics.com/news/Society-for-Neuroscience-49th-Annual-Meeting-Chicago.pdf
Discovery of PIPE-505, a small molecule therapeutic for the treatment of sensorineural hearing loss (SNHL) associated with cochlear synaptopathy
November 13, 2019