CATEGORY:
Research
SCREENSHOT:
TITLE:
A Selection Protocol to Identify Therapeutics to Target NLRP3-Associated Sensory Hearing Loss
CONTENT:
Otol Neurotol. 2024 Sep 6. doi: 10.1097/MAO.0000000000004321. Online ahead of print.
ABSTRACT
OBJECTIVE: We propose a selection process to identify a small molecule inhibitor to treat NLRP3-associated sensory hearing loss.
BACKGROUND: The NLRP3 inflammasome is an innate immune sensor and present in monocytes and macrophages. Once the inflammasome is activated, a cleavage cascade is initiated leading to the release of proinflammatory cytokines IL-1β and IL-18. The NLRP3 inflammasome has been implicated in many causes of hearing loss, including autoimmune disease, tumors, and chronic suppurative otitis media. Although the target has been identified, there is a lack of available therapeutics to treat NLRP3-associated hearing loss.
METHODS: We created a target product profile with specific characteristics that are required for a compound to treat sensory hearing loss. We then looked at available small molecule NLRP3 inhibitors at different stages of development and selected compounds that fit that profile best. Those compounds were then tested for cell toxicity in MTT assays to determine the dosage to be used for efficacy testing. We tested efficacy of a known NLRP3 inhibitor, MCC950, in a proof-of-concept screen on reporter monocytes.
RESULTS: Six compounds were selected that fulfilled our selection criteria for further testing. We found the maximum tolerated dose for each of those compounds that will be used for further efficacy testing. The proof-of-concept efficacy screen on reporter monocytes confirmed that those cells can be used for further efficacy testing.
CONCLUSION: Our selection process and preliminary results provide a promising concept to develop small molecule NLRP3 inhibitors to treat sensory hearing loss.
PMID:39284007 | DOI:10.1097/MAO.0000000000004321
SOURCE:
Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology
PUBLISHER:
PMID:
pubmed:39284007
ID:
0b58ea4968e09ff10f4e1238c494f316pubmed:39284007
DOI:
10.1097/MAO.0000000000004321
DATE – PUBLISHED:
Mon, 16 Sep 2024 06:00:00 -0400
DATE – DOI:
2024-09-16T20:00:43Z
DATE – ADDED:
09/17/24 10:43AM
LINK – PUBMED:
https://pubmed.ncbi.nlm.nih.gov/39284007/
LINK – DOI:
https://doi.org/10.1097/MAO.0000000000004321
LINK – PUBLISHER:
https://journals.lww.com/10.1097/MAO.0000000000004321?utm_source=hearinglosstreatmentreport.com
IMAGE:
REFERENCE:
Hearing Loss Treatment Report, Urgent Research, 2024-09-17T14:43:12+00:00, https://www.hearinglosstreatmentreport.com.
COMMENT: Why is this September 16, not September 6? All dates point to Sep 16, yet original article shows Sep 6……… comment from Michael Sutton Sep 18 12:48pm, regarding anomaly








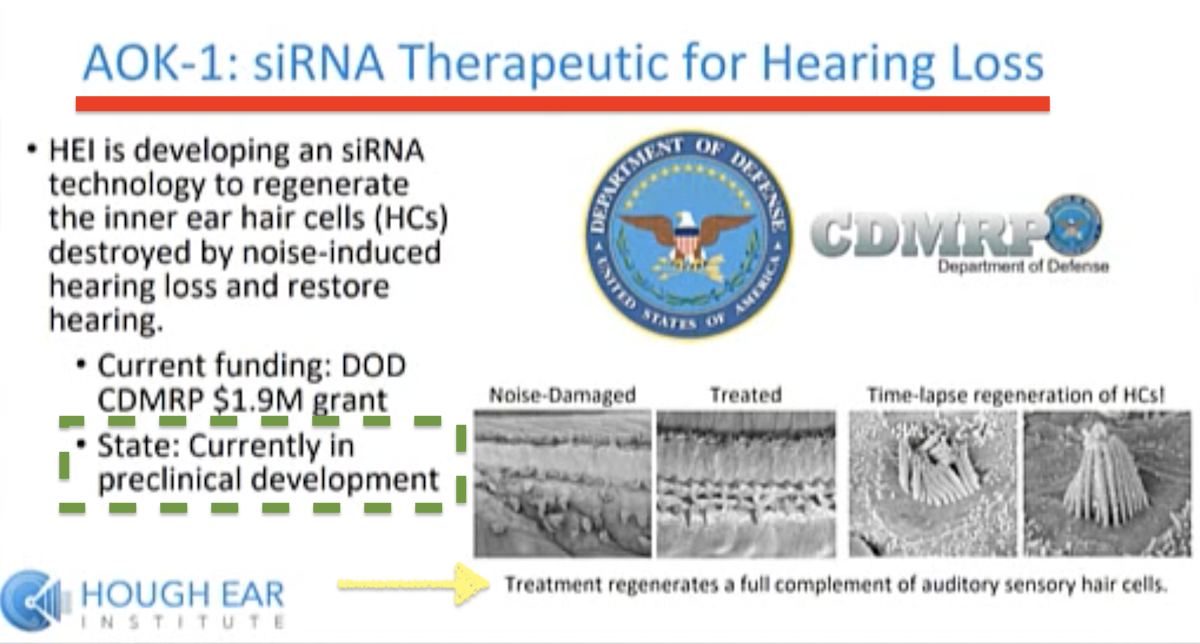
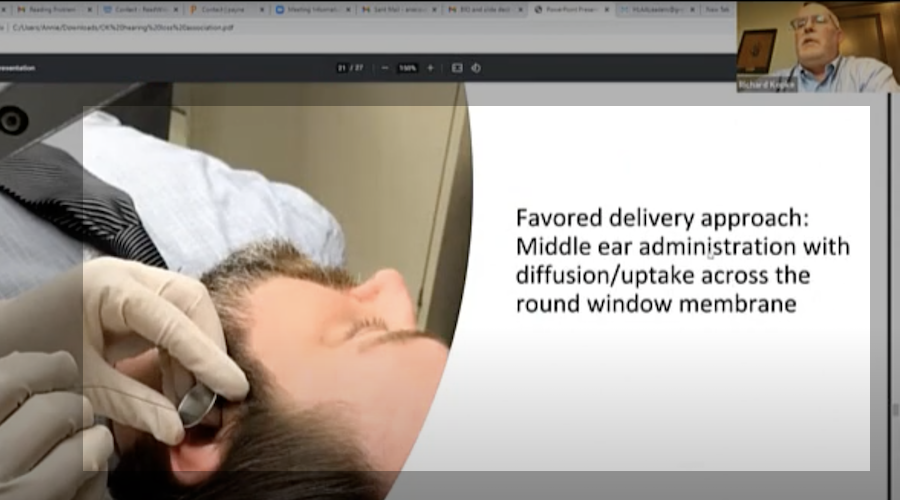
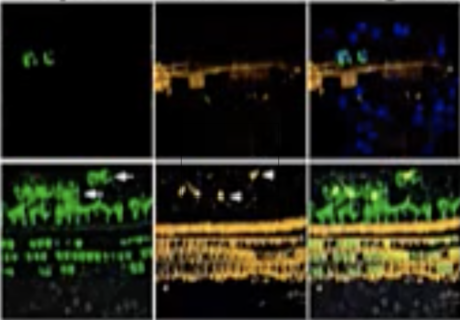
 What does that mean?
What does that mean?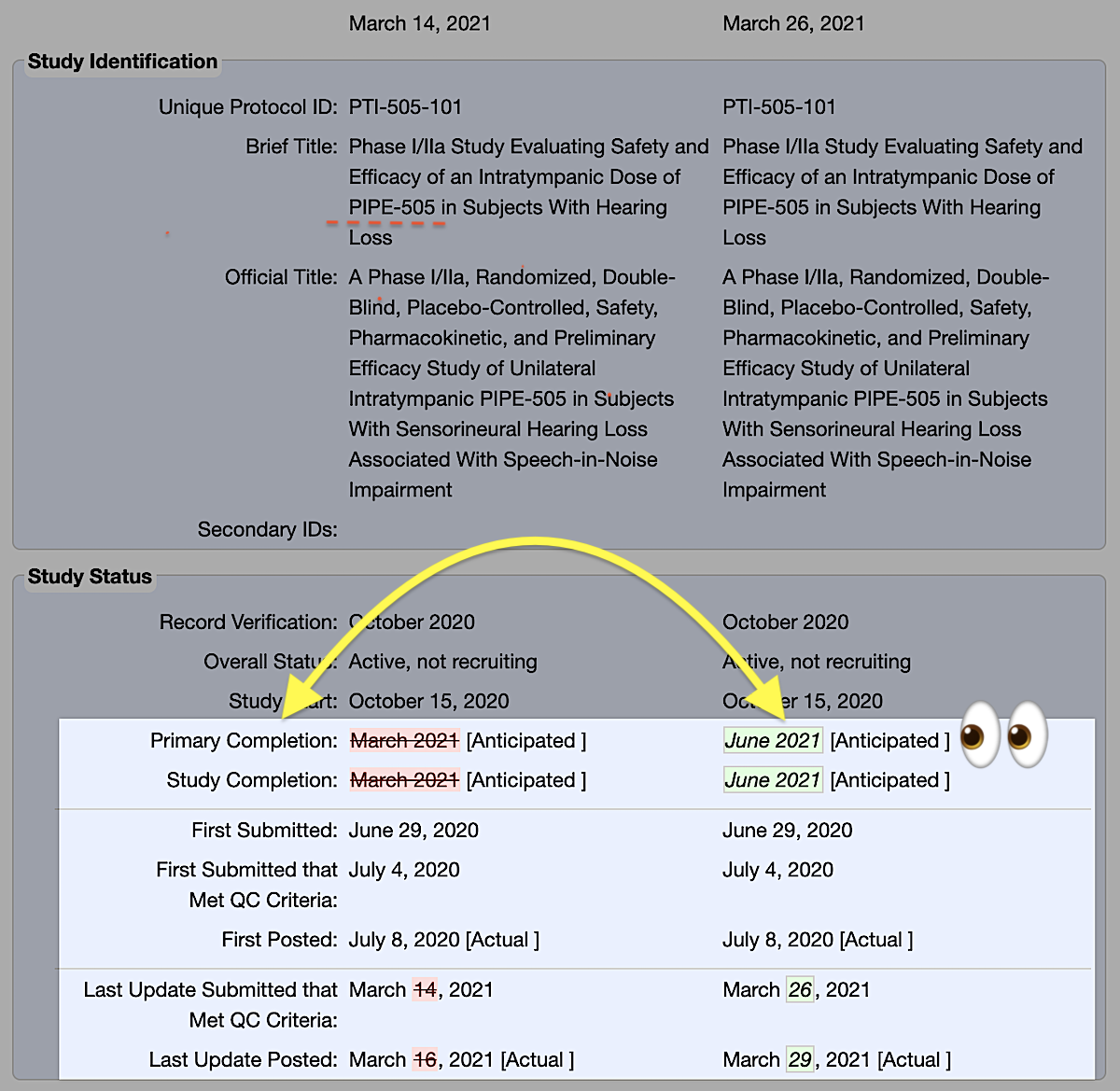
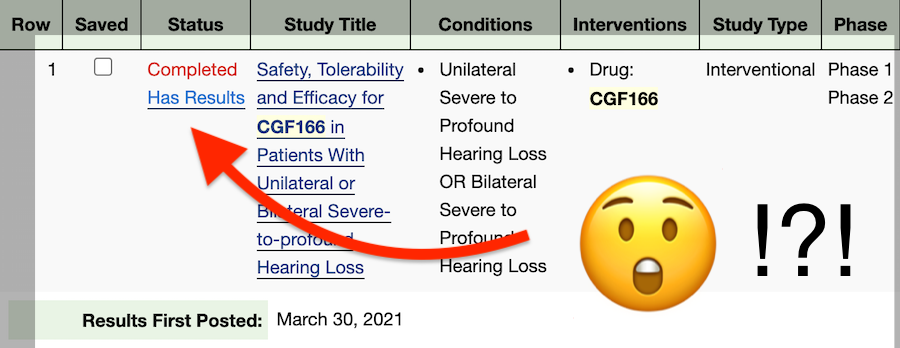
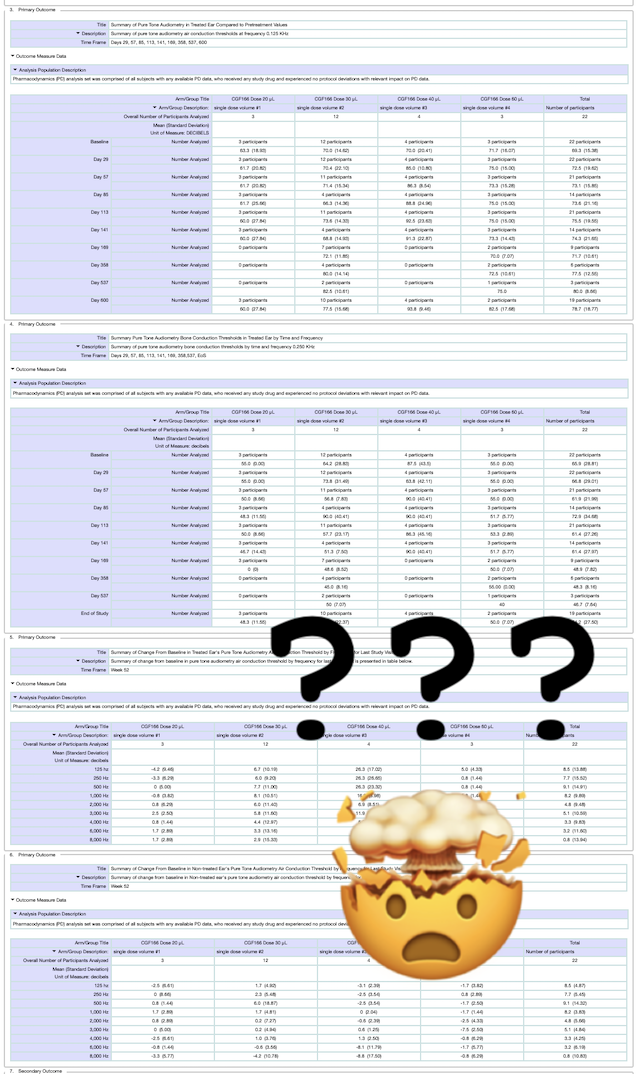 This can be frustrating, but here at Hearing Loss Treatment Report we are currently working on a follow-up post that will provide a clear idea of what these numbers mean for people with hearing loss.
This can be frustrating, but here at Hearing Loss Treatment Report we are currently working on a follow-up post that will provide a clear idea of what these numbers mean for people with hearing loss.