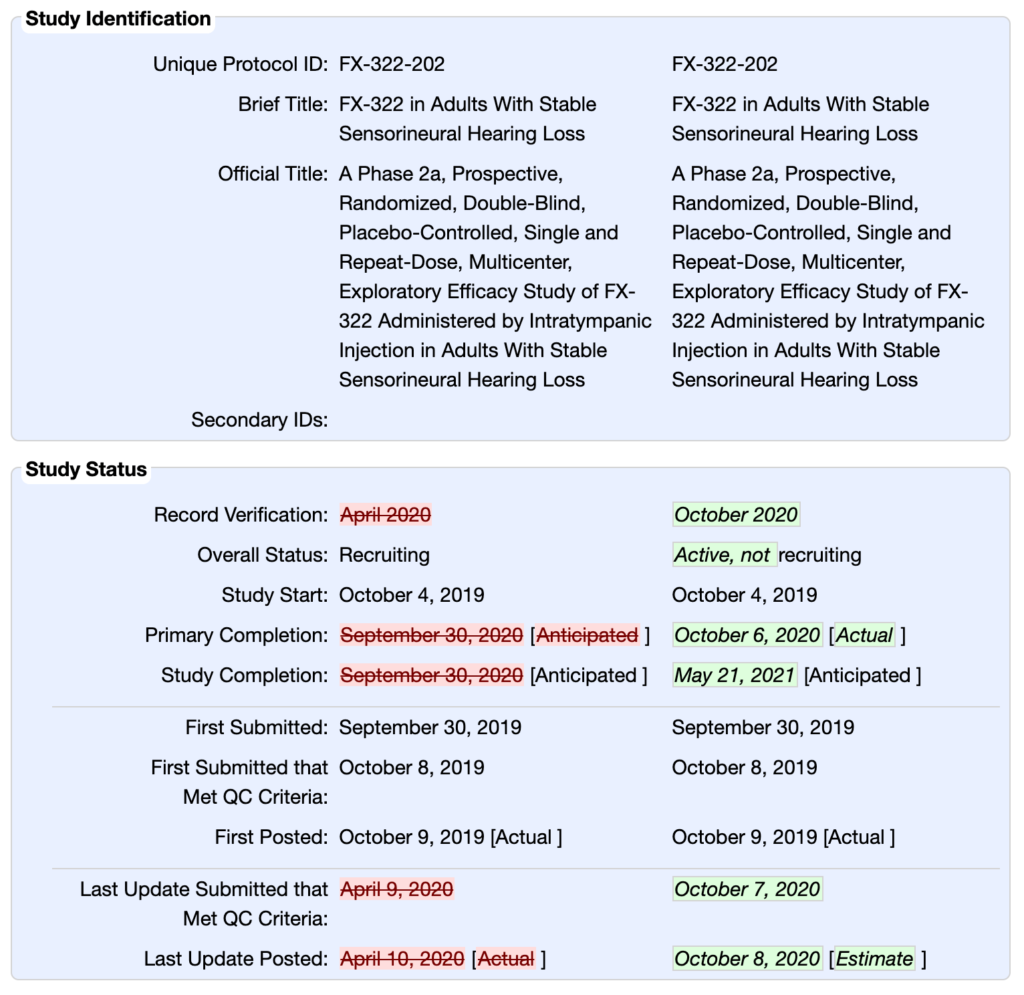
https://www.forbes.com/sites/williamhaseltine/2024/07/02/gene-therapy-to-restore-hearing-a-new-portal/
AUD1001 was shown to be safe and well tolerated, and provided early indications of efficacy in speech in noise, a key clinical outcome parameter. Importantly, the efficacy signals persisted a year after the treatment.
[…]Audion is preparing for initiation of new clinical trials of AUD1001 for sensorineural hearing loss at multiple sites in Europe and in the US in H2 2021. The planned Phase 2b double blind, placebo controlled trials will be conducted in adults with demonstrated loss of clarity, using a standardized local drug delivery.










 What does that mean?
What does that mean?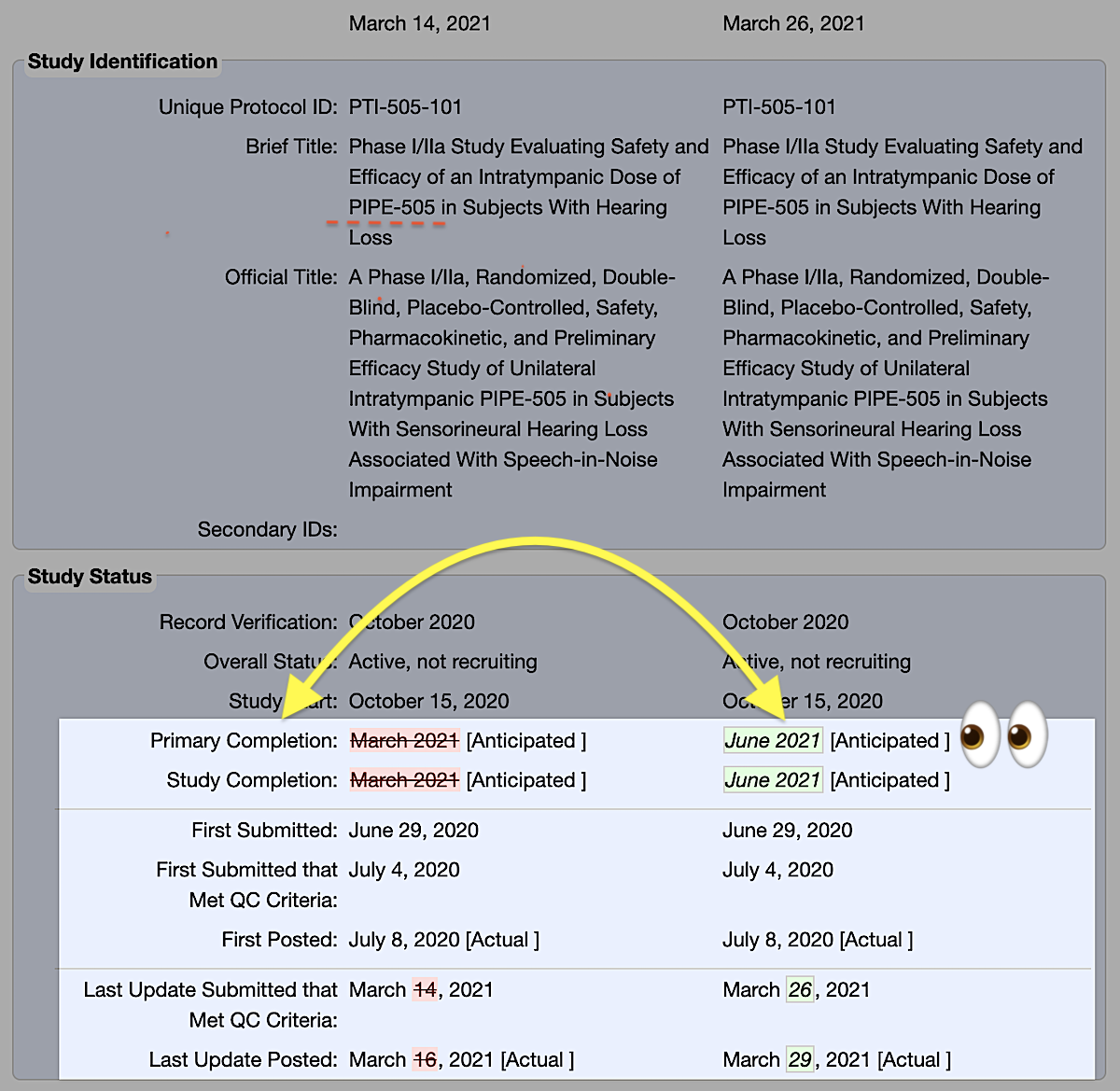
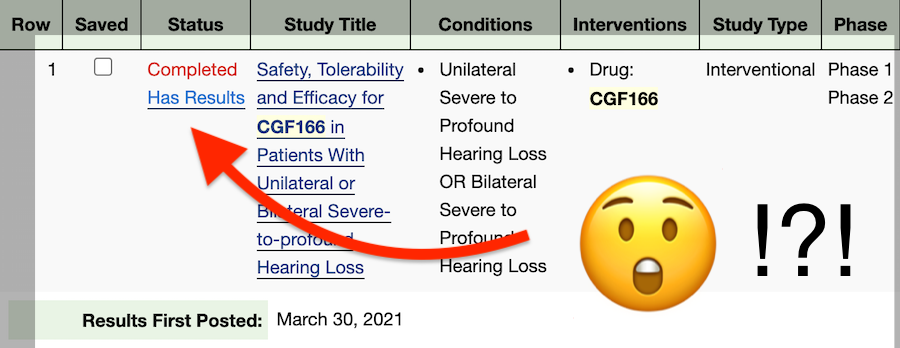
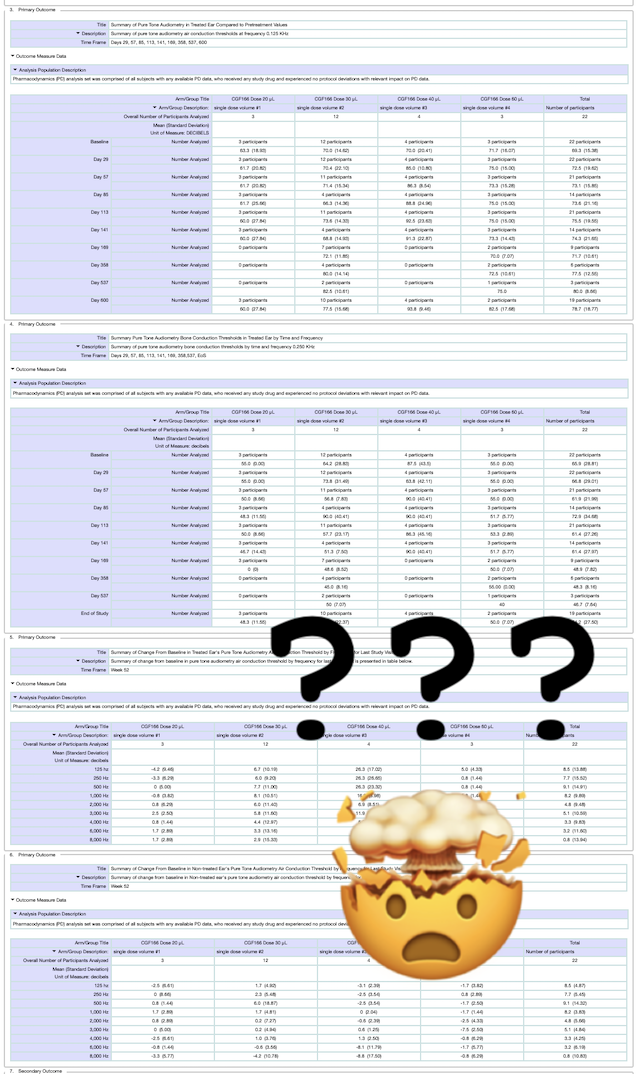 This can be frustrating, but here at Hearing Loss Treatment Report we are currently working on a follow-up post that will provide a clear idea of what these numbers mean for people with hearing loss.
This can be frustrating, but here at Hearing Loss Treatment Report we are currently working on a follow-up post that will provide a clear idea of what these numbers mean for people with hearing loss.